Session Information
Date: Monday, November 18, 2024
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Understanding the mechanisms that drive disease flares in patients with rheumatoid arthritis (RA) may aid in the development of biomarkers to facilitate targeted treatment withdrawal in patients in remission and may suggest therapeutic targets for flare prevention. However, these mechanisms are difficult to study due to the unpredictable nature of flare. The BIOlogical Factors that Limit sustAined Remission in rhEumatoid arthritis (BIO-FLARE) study1 is an experimental medicine study of ‘synchronised’ RA flares in which treatment is withdrawn in RA patients in clinical remission receiving conventional synthetic (cs)DMARDs. Subsequently, approximately 50% flare within 6 months, the remainder maintaining drug-free remission (DFR). We have assessed, longitudinally, circulating T cell subsets and mediators following treatment cessation, to better under the mechanisms driving flare and maintaining DFR.
Methods: Individuals with RA receiving csDMARDs, in clinician-defined remission with a DAS28-CRP < 2.4, were recruited into BIO-FLARE. Treatment was stopped and participants monitored over 24 weeks, with serum and Smart Tube-fixed peripheral blood stored at 0, 2, 5, 8, 12 and 24 weeks. Flare was defined as DAS28-CRP ≥3.2 at any study visit, or DAS28-CRP ≥2.4 on two occasions within a 14-day period. Circulating T cell subsets were assessed using a 16 marker Phosflow panel. Soluble serum mediators were detected by the NULISAseq™ Inflammation Panel 2502.
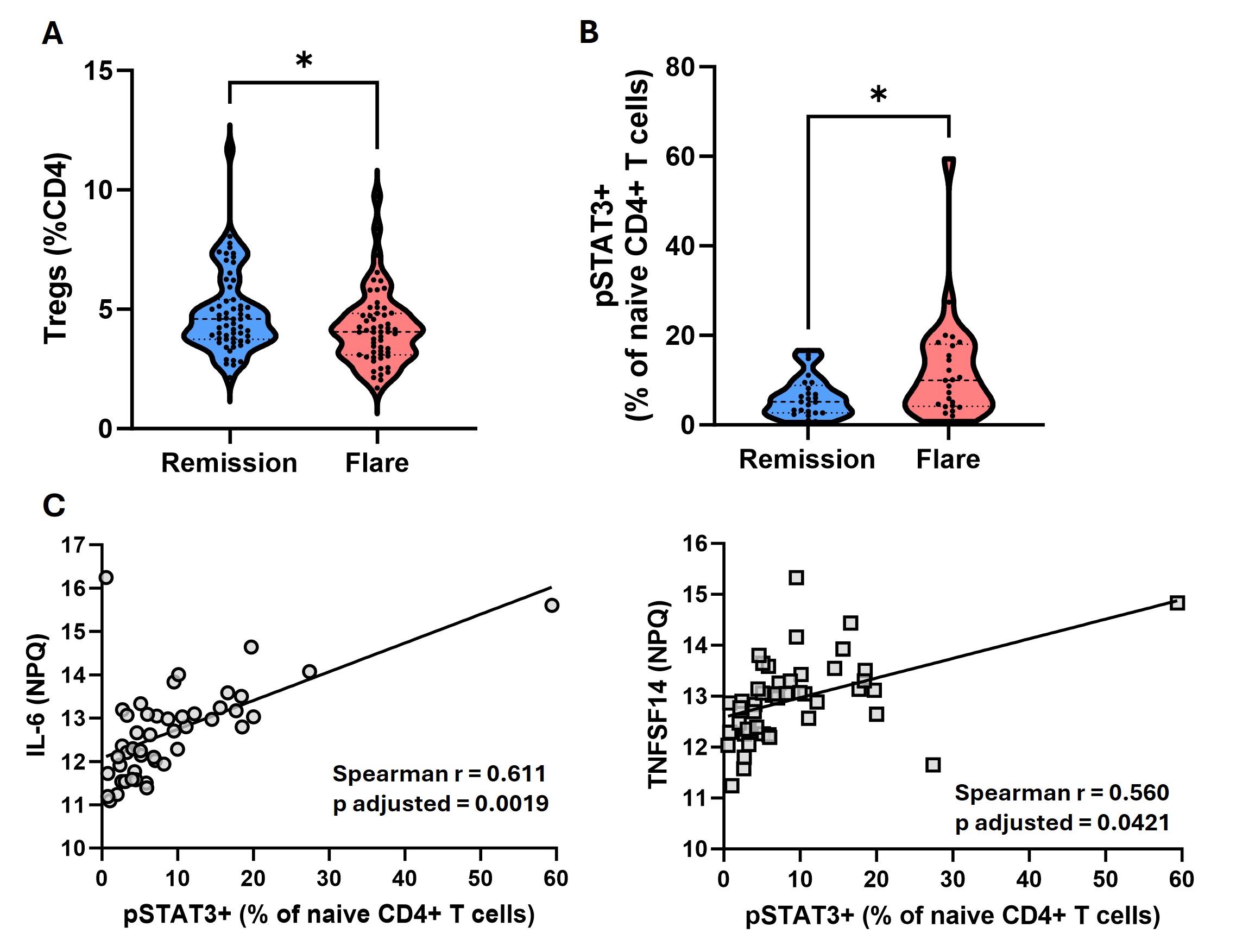
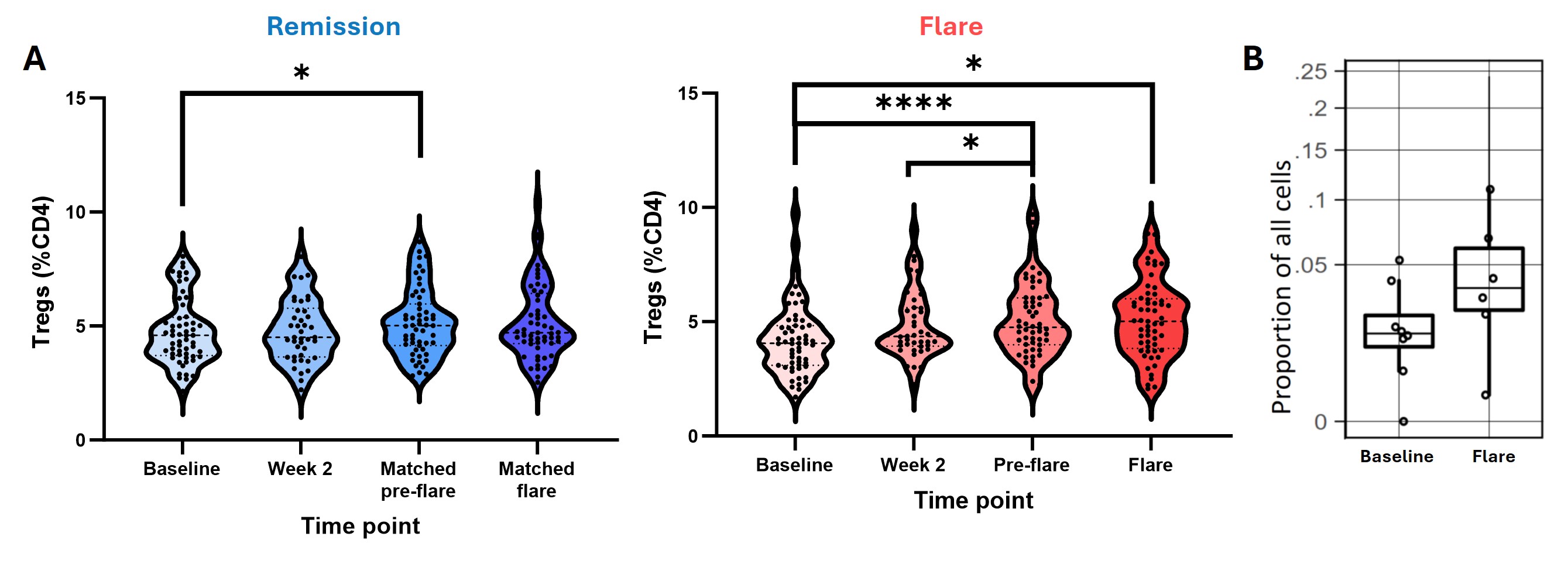
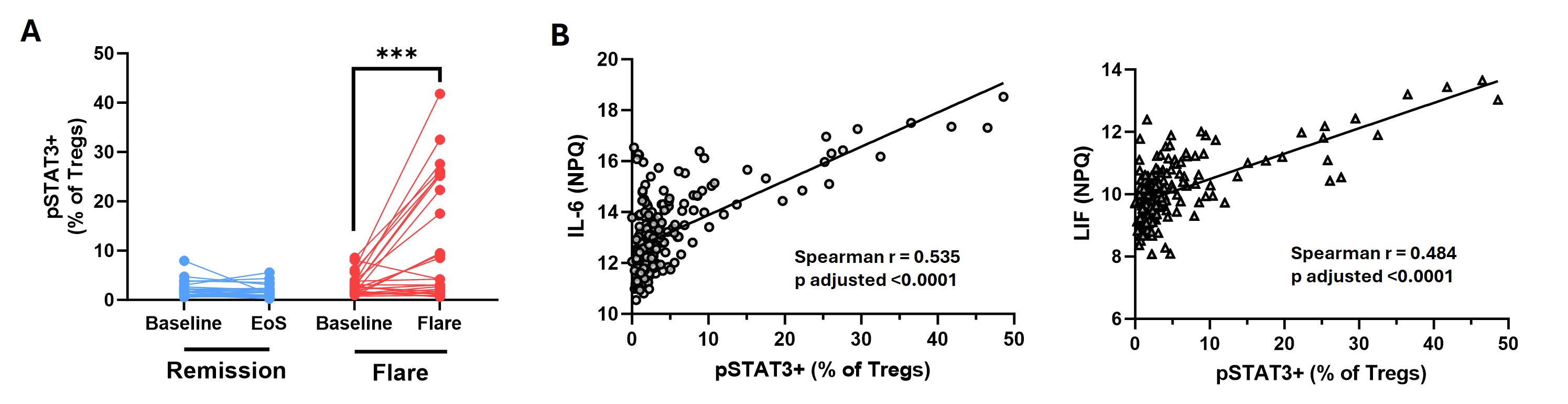
Results: At baseline, participants who subsequently flared and those who maintained DFR were well matched clinically, except for RF/ACPA titres, which were higher in those who subsequently flared. In contrast, flow cytometry analysis at baseline revealed an increase in the proportion of CD4+CD25high Tregs in the DFR group, as well as an increase in the proportion of naive CD4+ T cells expressing phosphorylated STAT3 (pSTAT3) in the flare group (Figure 1A-B). The proportion of pSTAT3+ naive CD4+ T cells strongly correlated with baseline levels of IL-6 and TNFSF14 (Figure 1C), despite similar levels of these cytokines in the two groups at baseline. When assessing longitudinal changes following DMARD withdrawal, Tregs increased in both groups (Figure 2A), as well as in the synovium of flare patients (Figure 2B). Expression of pSTAT3 in Tregs increased over time in the flare group only (Figure 3A) and correlated with IL-6 and LIF levels (Figure 3B).
Conclusion: Even at baseline there are elevated proportions of naive CD4+ T cells expressing pSTAT3 in patients who subsequently flare, and increased Treg proportions in those who achieve DFR. Whilst Treg proportions increase after DMARD withdrawal in both groups, those who flare have increased pSTAT3 expression in this subset, possibly driven by elevated circulating IL-6 and LIF. As pSTAT3 expression is suggested to impair Treg function, the Tregs expanded during flare may be dysfunctional due to their exposure to IL-6 and other pro-inflammatory cytokines. This work highlights key pathways involved in disease flare that warrant further investigation.
References
- Rayner F et al. BMC Rheumatol. 2021
- Feng W et al. Nat Commun. 2023
- Yang L et al. J Dermatol Sci. 2016
To cite this abstract in AMA style:
Anderson A, Jones L, Rayner F, Swift J, Maunder D, de Paula Lemos H, Swan D, Degnan A, Wilson I, Diboll J, Reynolds G, Sim J, Melville A, Siebert S, McInnes I, Goodyear C, Hilkens C, Raza K, Buckley C, Baker K, Pratt A, Filer A, Isaacs J. Treg Expansion and IL-6 Induced STAT3 Phosphorylation in CD4+ T Cells Is a Biomarker of Disease Flare in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/treg-expansion-and-il-6-induced-stat3-phosphorylation-in-cd4-t-cells-is-a-biomarker-of-disease-flare-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treg-expansion-and-il-6-induced-stat3-phosphorylation-in-cd4-t-cells-is-a-biomarker-of-disease-flare-in-rheumatoid-arthritis/