Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The application of single cell genomic approaches to the study of rheumatoid synovium has recently enabled scientists to study the complex relationship between different cell types and between cells and matrix in RA synovium. This brought new attention to the role of stromal cells in the joint such as fibroblast-like synoviocytes (FLS) and endothelial cells (EC). Recently it has been observed that FLS-like cells -called PRIME cells- are present in the circulation of RA patients suggesting that RA FLS can egress the RA synovium through blood vessels. However, trans-endothelial RA FLS migration has not yet been reported and its mechanisms remain unknown.
Methods: Trans-endothelial migration of FLS was assessed in RA synovium and in synovium of mice with serum transfer induced arthritis (STIA) by in situ hybridization (ISH) and immunofluorescence. Fluorescein-dextran was injected into mice for accessing vascular permeability. A FLS-EC transwell co-culture system in vitro was optimized and bulk RNA sequencing was performed on sorted FLS and EC.
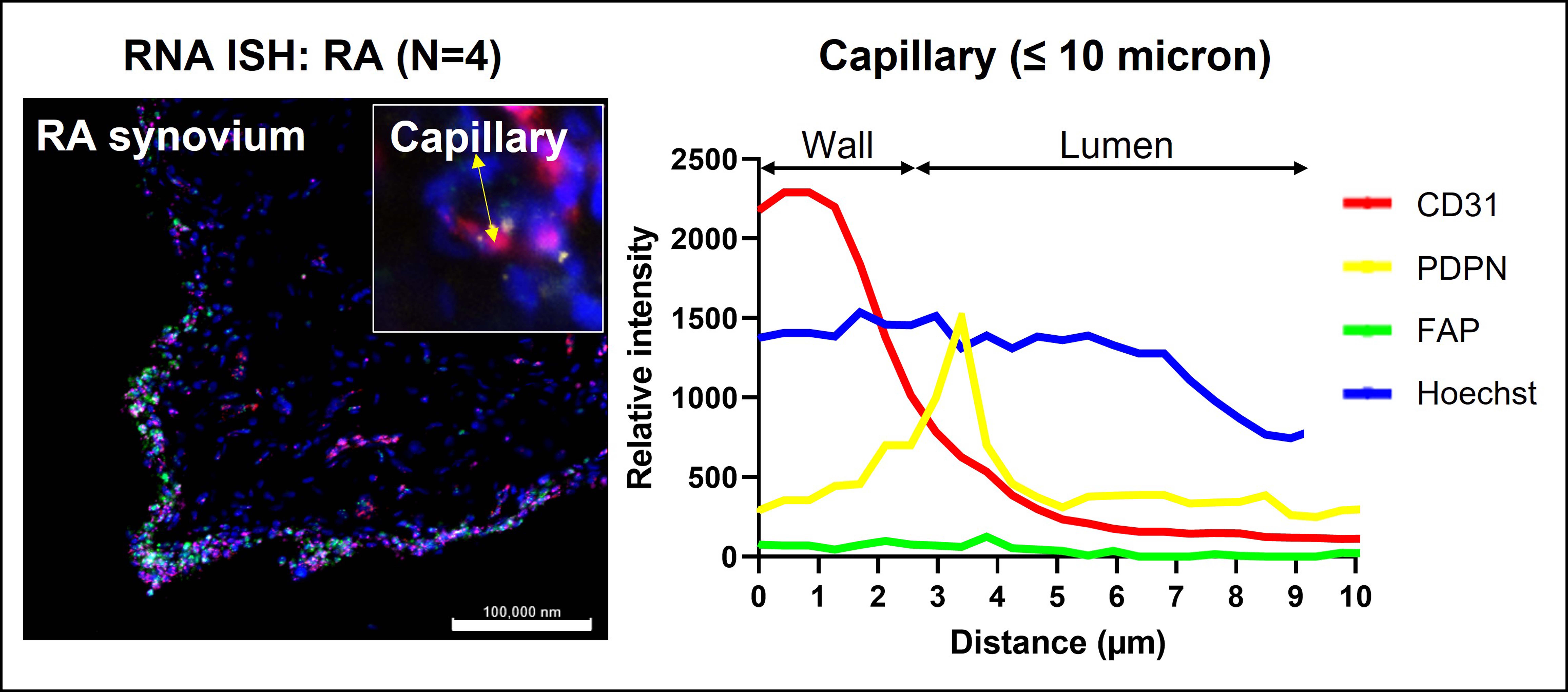
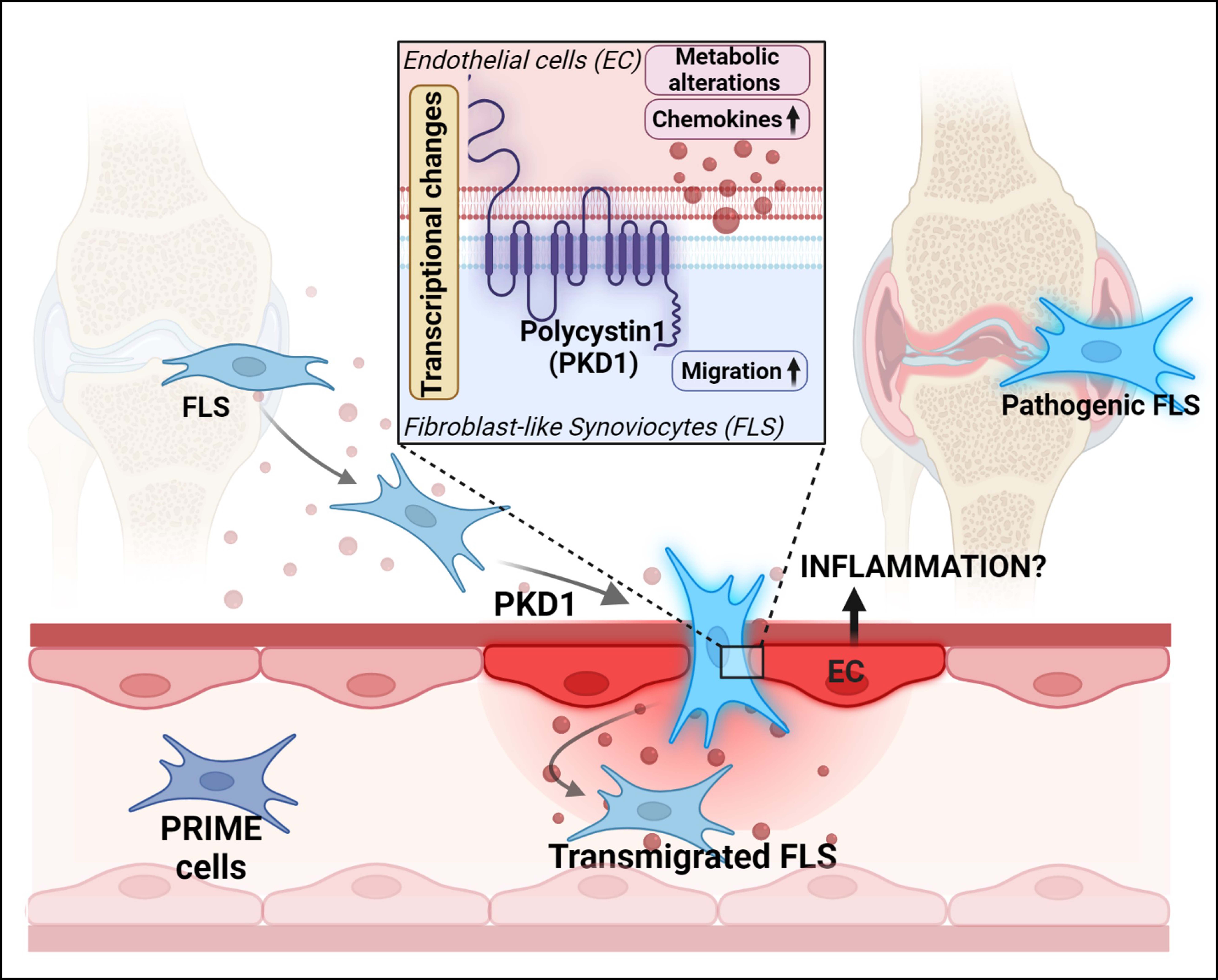
Results: We have collected initial evidence of FLS transmigration through endothelial layers in the synovium of RA patients and mouse STIA (Fig. 1). We next demonstrated that vascular FLS transmigration promotes vascular permeability in vivo. By leveraging a novel in vitro model of human RA FLS endothelial transmigration coupled with bulk RNA sequencing, we show that FLS transmigration not only is able to alter the permeability of EC layers, but also induces chemokines and other proinflammatory genes in EC and metabolic alterations in the EC layer. Trans-endothelial migration also induces expression of polycystin-1 in FLS, encoded by the PKD1 gene. We find that PKD1 serves as a selective promoter of endothelial transmigration of FLS and knockdown of PKD1 in FLS ameliorated arthritis in a mouse model.
Conclusion: Our data suggests the existence of a feedback loop triggered by FLS trans-endothelial migration that tends to perpetuate the process and enhances arthritis severity via induction of local inflammation, EC metabolic abnormalities, and generation of circulating FLS (Fig. 2). Our study contributes to elucidate the role of FLS migration in RA, highlights a novel angle of the FLS-EC interplay in rheumatoid synovium, and helps unravel the origin of PRIME cells in RA.
To cite this abstract in AMA style:
Kim J, Ainsworth R, Bottini N. Trans-endothelial Migration of Synovial Fibroblasts Promotes Arthritis Severity Through a PKD1-mediated Feedback Loop [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/trans-endothelial-migration-of-synovial-fibroblasts-promotes-arthritis-severity-through-a-pkd1-mediated-feedback-loop/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trans-endothelial-migration-of-synovial-fibroblasts-promotes-arthritis-severity-through-a-pkd1-mediated-feedback-loop/