Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Rheumatoid Arthritis (RA) patients treated with Janus Kinase inhibitors (JAKi) have reportedly higher venous thromboembolism (VTE) risk and atherosclerotic-related complications including myocardial infarction (1).Although underappreciated by Rheumatologists, in certain experimental situations JAK inhibition has been linked to increased pro-inflammatory cytokine production by myeloid cells(2,3). Thus, we hypothesized that JAK inhibition-related pro-inflammatory cytokine dysregulation with tofacitinib (JAKi) might actually drive “immunothrombosis” in both normal and RA blood.We thus investigated the circumstances where JAK inhibition may drive innate immune-mediated immunothrombosis
Methods: Blood was obtained from RA patients (n=12) under any treatment except JAKi and divided into two groups: remission/low disease activity (DAS-28-CRP < 3.2, n=6) or moderate/active disease activity (DAS-28-CRP ³ 3.2, n=6). Experiments were also performed on peripheral blood mononuclear cells (PBMCs) from healthy donors (n= 12) including other JAKi.Leukocytes were isolated using ammonium chloride and stimulated with TLR4 (lipopolysaccharide, LPS) and TLR3 (Polyinosinic:polycytidylic acid) agonists with and without tofacitinib at three pharmacologically relevant concentrations. Bulk RNA sequencing of stimulated cells, ELISA and Legendplex kits were used to assess cell responses to tofacitinib. Plasma turbidity assays of clot formation and fibrinolysis and thromboelastography assays were used to assess the dynamics of in-vitro clotting. Results were analyzed with GraphPad Prism software.
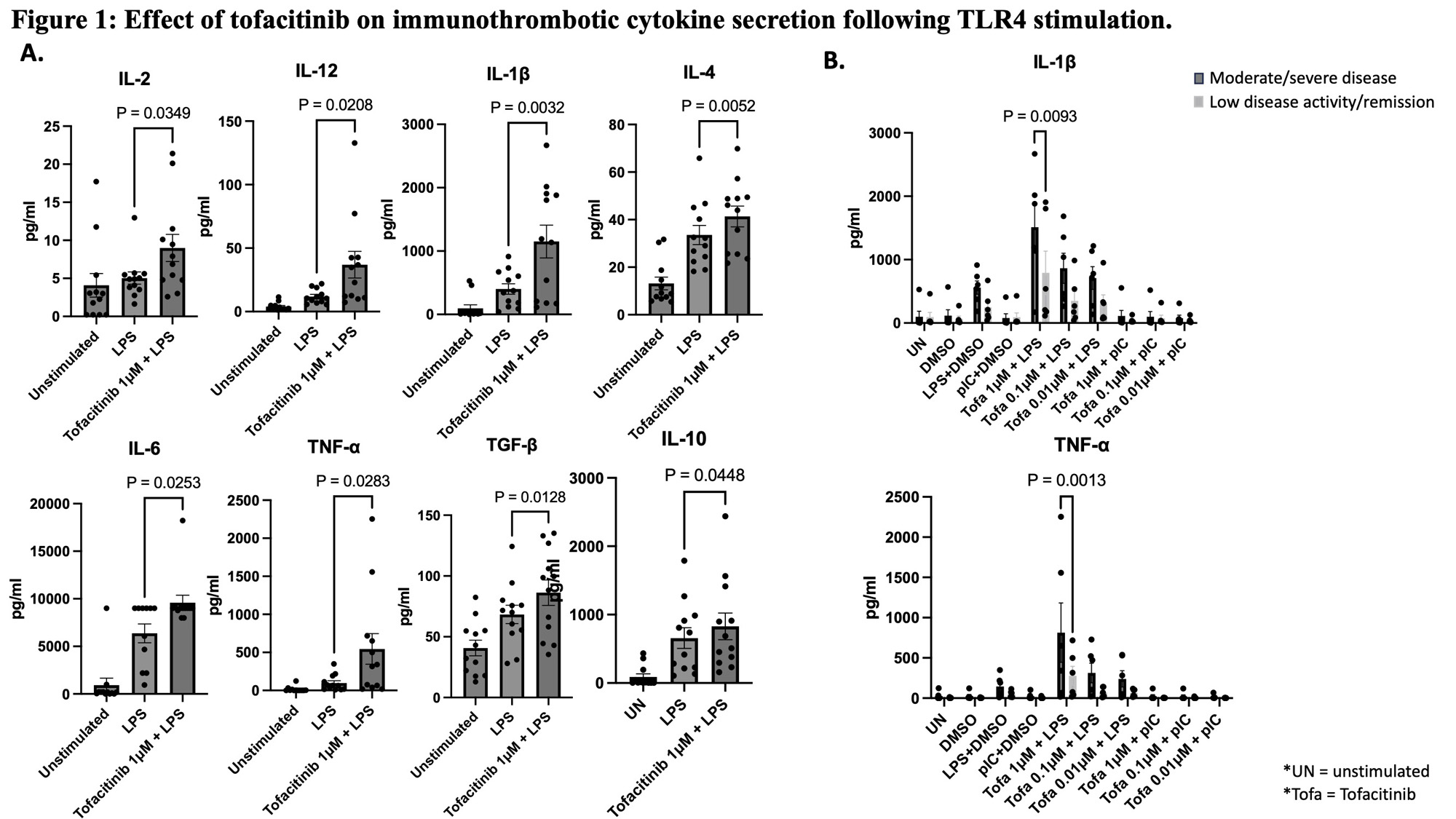
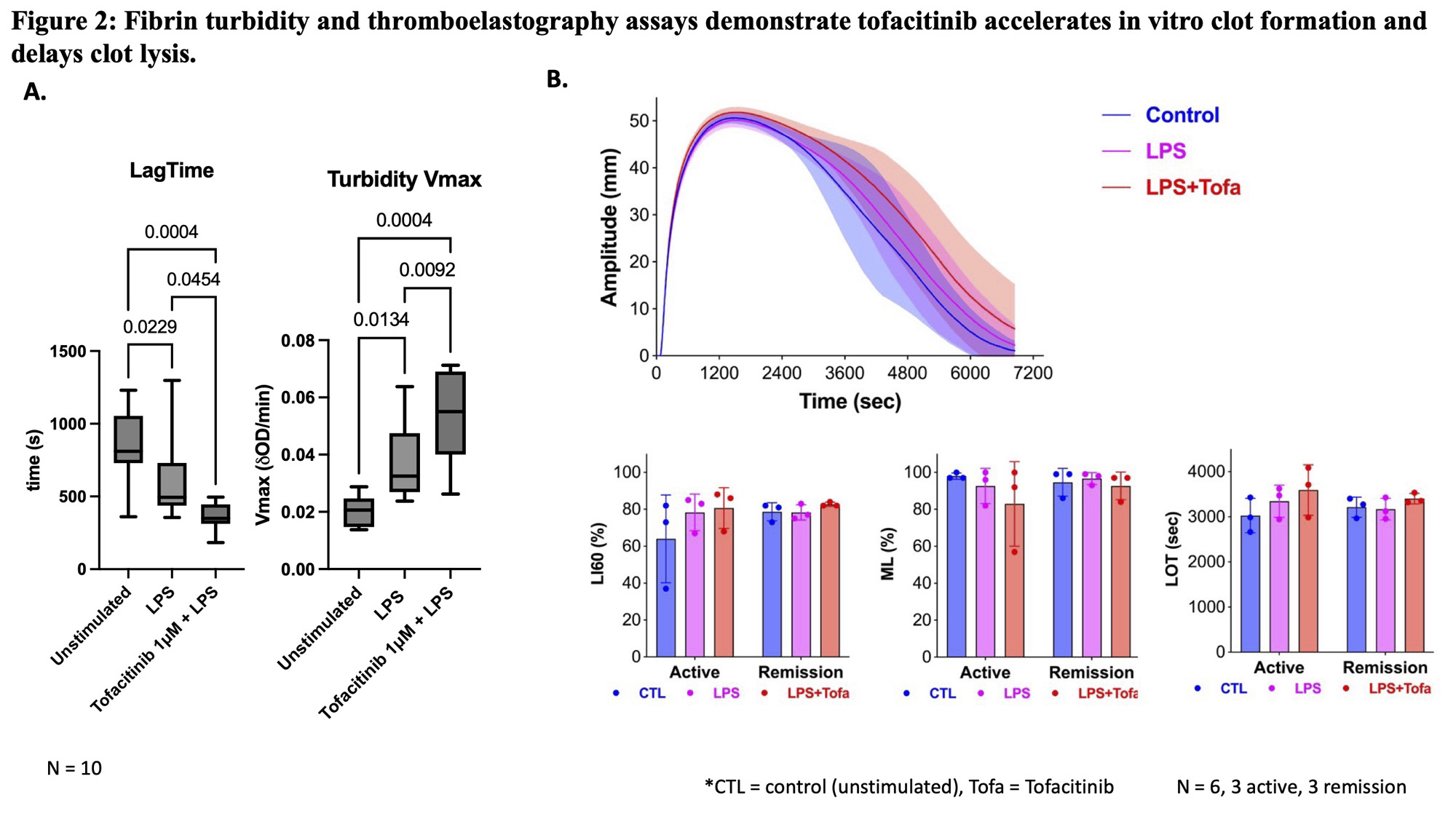
Results: In both RA and healthy blood, LPS-activated leukocytes treated with tofacitinib at therapeutically relevant concentrations (1 mM, 0.1 mM and 0.01 mM) further increased cytokines associated with immunothrombosis, including TNF-α, IL-1β, IL-12, IL-4, IL-6 and TGF-b (Fig 1A). This effect was absent with TLR3 agonists (not shown), and more evident in active RA when compared to remission (Fig 1B) despite similar baseline cytokine levels. Bulk RNA sequencing data showed multiple pathways whereby tofacitinib augmented LPS-treated macrophage inflammatory signatures including the NF-kB pathway, IL-12, and IL-23 pathway, and suggested disruption of immunoregulatory interferon- and IL-10-induced pathways.In turbidity and lysis analysis, all LPS-activated cell supernatants induced faster clot formation than the buffer control. However, clot formation rate was further increased significantly when tofacitinib was added in combination with LPS (Fig 2A).Thromboelastography showed delayed clot lysis in active RA patients’ blood treated with LPS/tofacitinib, but not in remission/low disease activity subjects (Fig 2B).
Conclusion: TLR4, but not TLR3, activated myeloid cells treated with JAKi exhibited accelerated clot formation and delayed thrombolysis in RA blood samples in-vitro. This work offers novel insights into how venous and arterial immunothrombosis might occur in RA, especially in the context of bacterial challenge in active RA on JAKi therapy.
References
1 Molander V et al. Ann Rheum Dis. 2022
2 Pattison MJ et al. The Journal of Immunology. 2012
3 Chen F et al. J Immunol. 2016
To cite this abstract in AMA style:
David P, Macleod T, Shi Y, Ganguly P, Duval C, Harland M, Wong C, Saleem B, McGonagle D. Tofacitinib Accelerates in Vitro Clot Formation and Delays Clot Lysis in Rheumatoid Arthritis Blood by Enhancement of Macrophage TLR4 Mediated Cytokine Responses- a New Angle on Thrombosis with JAKi in Rheumatology [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/tofacitinib-accelerates-in-vitro-clot-formation-and-delays-clot-lysis-in-rheumatoid-arthritis-blood-by-enhancement-of-macrophage-tlr4-mediated-cytokine-responses-a-new-angle-on-thrombosis-with-jaki-i/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tofacitinib-accelerates-in-vitro-clot-formation-and-delays-clot-lysis-in-rheumatoid-arthritis-blood-by-enhancement-of-macrophage-tlr4-mediated-cytokine-responses-a-new-angle-on-thrombosis-with-jaki-i/