Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that leads to progressive joint destruction involving multiple joints. There is a large body of evidence that suggests a crucial role for activated synovial fibroblasts in mediating both direct tissue injury and perpetuation of the complex disease process in RA (1). Synovial fibroblasts derived from RA patients (RASF) are able to attach to articular cartilage and deeply invade and degrade the cartilage matrix (2) and have been shown to migrate to secondary joint locations in vivo and invade and degrade cartilage similarly to the primary site (3). Human gingival-derived mesenchymal stem cells (GMSC) are promising therapeutic cell treatments for autoimmune diseases due to their immunomodulatory capacity (4). Others have demonstrated the ability of GMSC, and exosomes derived from GMSCs (GMSCExo) to suppress the deleterious in vivo effects of the collagen induced arthritis model in mice (5). Our aim was to test whether the destructive invasive effects of RASF in an in vivo chimeric mouse model of RA could be modulated by the presence of GMSC or GMSCExo. In addition, we analyzed the effects of both GMSC and GMSCExo on the ability of RASF to migrate to secondary locations. Mechanistic studies were done to understand how the GMSC were inhibiting the invasiveness of the RASF.
Methods: A chimeric human/mouse model of synovitis was created by surgically implanting SCID mice with a small piece of human articular cartilage surrounded by RASF. Each mouse received two implants; the primary implant on the right flank of the mouse contained RASF, the secondary implant contained no RASF. Mice were retro-orbitally injected once with either GMSC or GMSCExo at 5-7 days post-implantation. The implants were removed after 60 days for evaluation. Histology and IHC were used to assess RASF invasion of the cartilage. Flow cytometry was used to understand the homing ability of GMSC in vivo, the incidence of apoptosis of RASF and the exchange of exosomes in vitro.
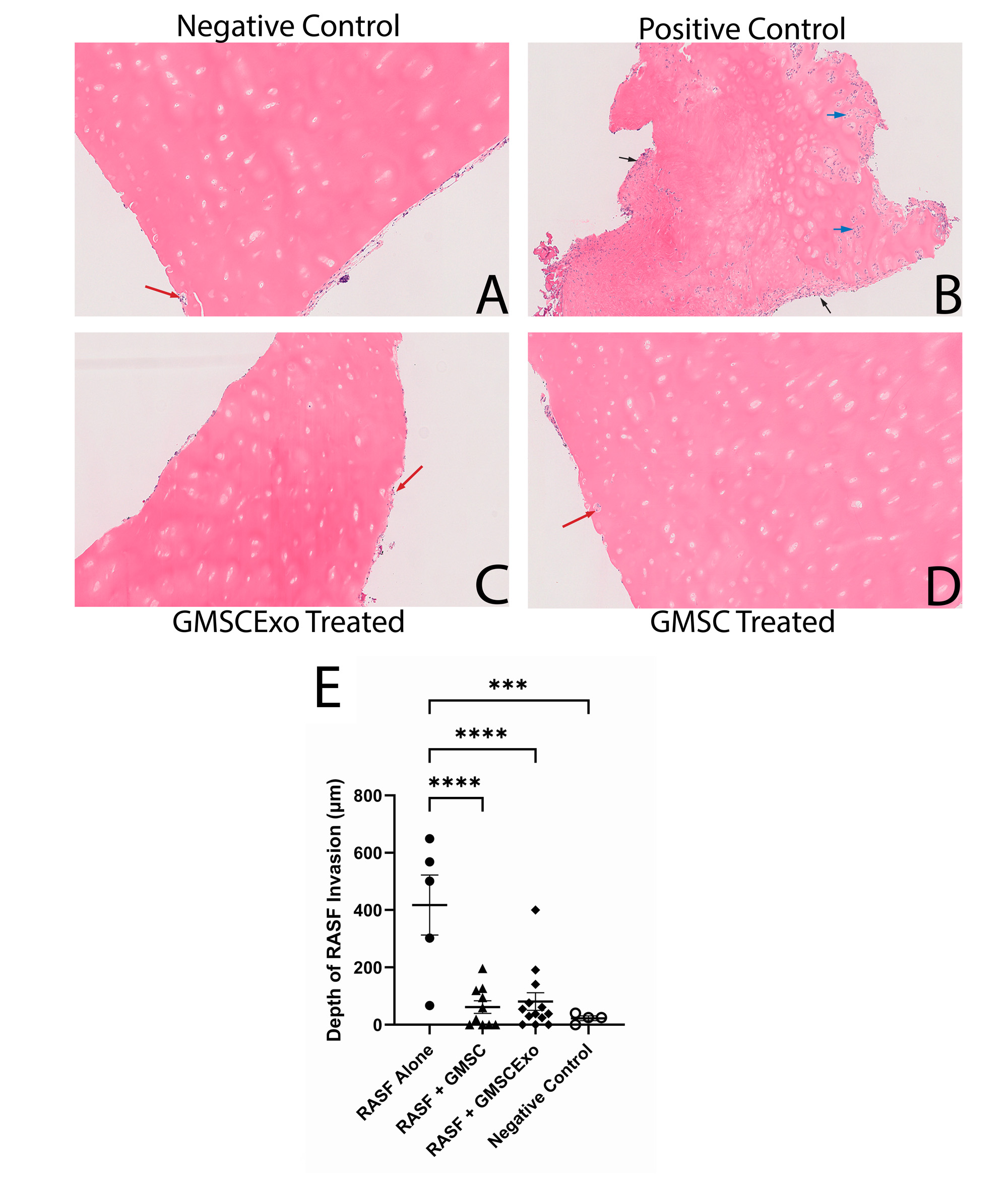
Results: We demonstrate that both GMSC and GMSCExo are potent inhibitors of the deleterious effects of RASF. Both treatments were effective in inhibiting the invasive destructive properties of RASF as well as the potential of these cells to migrate to secondary locations and attack the cartilage there. We also present evidence that GMSC home to the site of the implant and induce programmed cell death of the RASF through the direct transfer of exosomes.
Conclusion: Our results indicate that both GMSC and GMSCExo can block the pathological effects of RASF in this chimeric model of RA. A single dose of either GMSC or GMSCExo can inhibit the deleterious effects of RASF. These treatments can also block the invasive migration of the RASF, suggesting that they can inhibit the spread of RA to other joints. Because the gingival tissue is harvested with little difficulty, relatively small amounts of tissue are required to expand the cells, the fairly simple in vitro expansion process, and the increasing technological advances in the production of therapeutic exosomes, we believe that GMSC and GMSCExo are excellent candidates as a treatment for RA.
(A) Untreated cartilage showing no RASF invasion (B) RASF treated cartilage showing perichondrocytic invasion, blue arrows, and pannus like invasive structures degrading the cartilage (C) RASF treated followed by GMSCExo treatment blocks RASF invasion and degradation (D) RASF treated followed by GMSC treatment blocks RASF invasion and degradation (E) GMSCExo and GMSC both block RASF cartilage invasion
To cite this abstract in AMA style:
Bruckner s, Zeno B, Capria V, Willis W, Ganesan L, Jarjour w. The Therapeutic Effects of Gingival Mesenchymal Stem Cells and Their Exosomes in a Chimeric Model of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-therapeutic-effects-of-gingival-mesenchymal-stem-cells-and-their-exosomes-in-a-chimeric-model-of-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-therapeutic-effects-of-gingival-mesenchymal-stem-cells-and-their-exosomes-in-a-chimeric-model-of-rheumatoid-arthritis/