Session Information
Date: Tuesday, November 14, 2023
Title: Plenary III
Session Type: Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: In healthy synovium, lining fibroblasts promote joint health by secreting proteoglycans that lubricate the joint cavity. In rheumatoid arthritis (RA), sublining fibroblasts undergo marked expansion while lining fibroblasts are relatively diminished1–4. We have previously demonstrated the role of vascular endothelial-derived Notch signaling as a driver of perivascular sublining fibroblast differentiation4. Here, we seek to identify the transcriptional regulators of synovial lining fibroblast differentiation using synovial patient-derived organoids.
Methods: We developed two 3D tissue culture approaches to study fibroblast differentiation. First, we developed patient-derived organoids from synovial tissue explants that maintain fibroblast heterogeneity ex vivo. We leveraged an in vitro micromass system5 to examine the stability and reversibility of fibroblast differentiation. Fibroblast differentiation in organoids were examined via confocal microscopy, histology, and single-cell RNA sequencing (scRNAseq). A targeted transcription factor screen was performed using siRNA and CRISPR gene-editing to identify candidate transcription factors driving fibroblast differentiation.
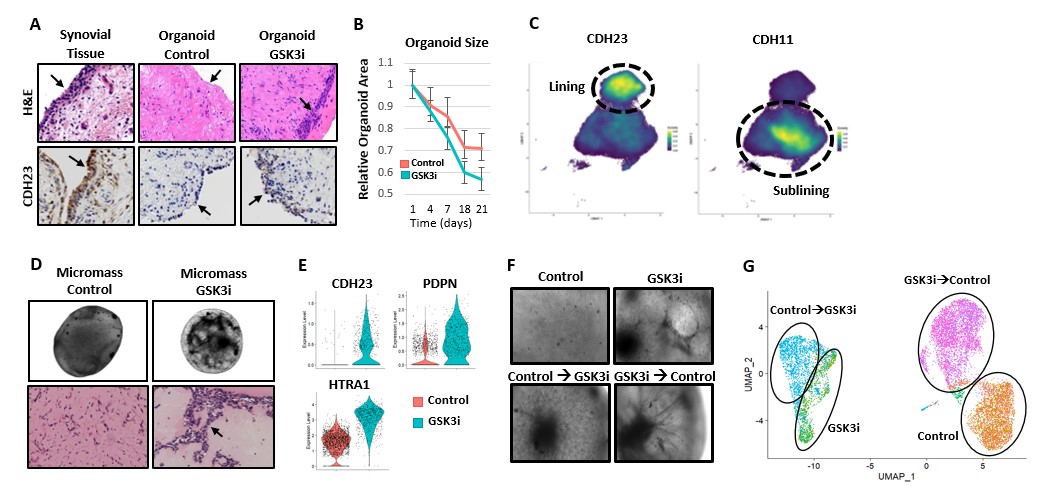
Results: Addition of a GSK3 inhibitor leads to compaction within patient-derived organoids, resembling a synovial lining-like layer (Fig 1A-B). We identified cadherin-23 (CDH23), an atypical cadherin, as a novel cadherin that is specific to lining fibroblasts (Fig 1C). Inhibition of GSK3 results in profound changes in gene expression, including upregulation of CDH23 and other lining fibroblast marker genes (Fig 1E). This GSK3i-induced gene expression program is reversible suggesting active signaling is required to maintain this phenotype (Fig 1F-G). We performed a targeted screen against transcription factors that are induced by GSK3 inhibition. This screen identified β-catenin as a necessary factor for mediating key transcriptional changes consistent with a lining-like phenotype in synovial fibroblasts (Fig 2A). Consistent with β-catenin’s potential role as a co-transcription factor, we observed increased β-catenin expression and nuclear localization in GSK3i-treated fibroblasts (Fig 2B-C).
Conclusion: Inhibition of GSK3 induces a reversible lining-like phenotype in synovial fibroblasts. β-catenin is necessary for upregulation of lining fibroblast marker genes in the context of GSK3 inhibition. Further work is required to confirm direct transcriptional effects of β-catenin on synovial fibroblasts and upstream mechanisms. Understanding the mechanism underlying fibroblast differentiation could lead to identification of novel therapeutic targets for restoring synovial lining function in RA patients.
1. F. Mizoguchi et al., Nat. Commun.9, 789 (2018).
2. F. Zhang et al., Nat. Immunol.20, 928–942 (2019).
3. A. P. Croft et al., Nature. 570, 246–251 (2019).
4. K. Wei et al., Nature. 582, 259–264 (2020).
5. H. P. Kiener et al., Arthritis Rheum.62, 742–752 (2010).
(A) IHC staining of synovial organoids displaying compaction and upregulation of CDH23 when treated with GSK3i, which resembles synovial lining. (B) Organoid size decreases more drastically when exposed to GSK3i indicating a compaction phenotype (C) Density plot of CDH23 and CDH11 mRNA expression in synovial fibroblasts from the Accelerating Medicines Partnership scRNAseq dataset (D) Micromass generated from synovial fibroblasts. (E) RNA expression of lining marker genes upregulated by GSK3 inhibition. (F) Live imaging of micromass that were either grown in control media, exposed to GSK3i, exposed to GSK3i after initially grown in control media, or grown in control media after initially exposed to GSK3i. (G) UMAP depicting scRNAseq data on micromass fibroblasts showing partial reversibility of GSK3i-induced transcriptional profile.
(A) Normalized mRNA expression of CDH23 with siRNA knockdown against CTNNB1, which encodes β-catenin. (B) Immunofluorescent images of β-catenin (green) in synovial fibroblasts treated with either a GSK3 inhibitor or CTNNB1 siRNA. (C) Western blot showing upregulation of β-catenin in synovial fibroblasts treated with GSK3i. (D) High expression of β-catenin detected in synovial lining.
To cite this abstract in AMA style:
Presti S, Watts G, Case J, Zhu Z, Bowman T, Nguyen H, Nigrovic G, Gao C, Bhamidipati K, Li Y, Kongthong S, Zhang F, Jonsson A, (AMP RA/SLE) Network A, BWH S, Brenner M, Kazerounian S, Wei K. The Role of β-catenin in Synovial Lining Fibroblast Differentiation [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-role-of-%ce%b2-catenin-in-synovial-lining-fibroblast-differentiation/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-role-of-%ce%b2-catenin-in-synovial-lining-fibroblast-differentiation/