Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: RA is an inflammatory disease that results in pain and loss of function, especially in the hands and wrists. Brief self-assessment tools that can reliably and precisely quantify hand/wrist function are needed to assess inflammatory activity when a physical exam is not feasible and to capture day-to-day experience of living with RA. Neuro-QoL is part of the PROMIS family of self-report measures created using a patient-centred approach and IRT methodology. The Neuro-Qol Upper Extremity Function (UEF) scale measures ability across fine motor and ADLs involving digital, manual and reach-related function and self-care. Little is known about its performance in RA. Our goal was to evaluate the validity and responsiveness of the 8-item Neuro-QoL UEF in adults with RA and compare its performance with legacy measures of physical function. We hypothesized scores would be strongly (r >0.7) associated with MHAQ, MD-HAQ, and PROMIS PF, and moderately (r=0.4 to 0.7) with symptoms, disease activity, and QoL indicators, and be responsive to change in disease activity and PF.
Methods: Data were from the 0 and 6-month visits of adults with early RA (symptoms < 1 yr) enrolled in the Canadian Early Arthritis Cohort, a prospective real-world study at 16 sites across Canada. Participants completed the Neuro-QoL UEF, MHAQ, MDHAQ, PROMIS-29, and PT Global at each visit. Rheumatologists recorded joint counts and MD Global. To evaluate content validity, we examined descriptive statistics across CDAI disease activity levels, and Pearson correlations between the Neuro-QOL UEF, legacy measures, CRP & ESR. Responsiveness was assessed by correlating change scores from visits 0-6 between Neuro-QoL UEF, disease activity and legacy PF scores.
Results: The 262 participants were mostly white (83%) women (71%) with a mean (SD) age of 55 (13). Summary statistics at 6-months are shown in Table 1. Neuro-QOL UEF was moderately-strongly correlated with MHAQ, MDHAQ, PROMIS-PF (|r|=0.63-0.75) and moderately correlated with pain and stiffness, (|r|=0.59, -0.64), and CDAI, SDAI, Patient & MD Global, TJ & SJ (|r|=0.39-0.58). Neuro-QOL UEF was moderately correlated with PROMIS QoL domains Pain, Fatigue, Anxiety, Depression, Sleep & Participation (|r|=0.39-0.60).
Neuro-QOL scores decreased in a dose-response manner across worsening CDAI DA states reflecting increasing impairment (Table 2). Persons with HDA reported the highest disability, scoring nearly 0.5 SD lower on the Neuro-QoL UEF than PROMIS PF. Change from baseline to 6 months in Neuro-QoL UEF was moderately correlated with changes in PROMIS PF, MHAQ, Patient Global, and CDAI (|r|=0.44-0.65). The mean change and range from 0-6 months in Neuro-QoL was significantly larger than in PROMIS (8.9 [95% CI 7.5, 10.4] vs. 5.4 [95% CI 4.4, 6.4])(see Figure).
Conclusion: Clinicians, researchers, and patients benefit from practical self-report tools that reliably and precisely monitor hand function in RA. Results offer initial evidence of validity and responsiveness and support use of Neuro-QoL UEF to self-assess inflammatory activity in the hands and day-to-day experiences of living with RA.
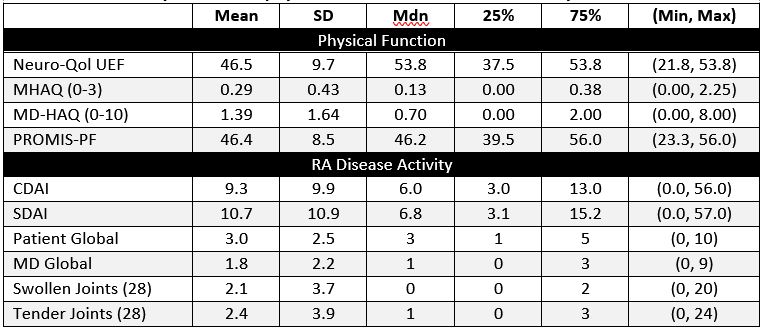 Table 1. Summary statistics of physical function and RA disease activity indices at 6 months.
Table 1. Summary statistics of physical function and RA disease activity indices at 6 months.
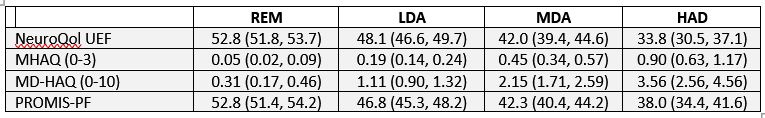 Table 2. Mean scores (95% CI) at 6 months by CDAI level.
Table 2. Mean scores (95% CI) at 6 months by CDAI level.
To cite this abstract in AMA style:
Bartlett S, Schieir O, Valois M, Pope J, Boire G, Keystone E, Tin D, Thorne C, Hitchon C, Bessette L, Hazlewood G, Bykerk V, Investigators C. The Neuro-QOL Upper Extremity Function Scale: New Opportunities to More Reliably and Precisely Measure Self-reported Hand Function and Self-care Activities in People with RA [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-neuro-qol-upper-extremity-function-scale-new-opportunities-to-more-reliably-and-precisely-measure-self-reported-hand-function-and-self-care-activities-in-people-with-ra/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-neuro-qol-upper-extremity-function-scale-new-opportunities-to-more-reliably-and-precisely-measure-self-reported-hand-function-and-self-care-activities-in-people-with-ra/

