Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation of the synovium. Despite a variety of treatments, 30-40% of RA patients fail to respond to any of the approved treatment options. Thus, improving the understanding of the molecular background and complex disease mechanisms in RA is of utmost importance.
Programmed death 1 (PD-1) is an inhibitory glycosylated immune checkpoint receptor that dampens immune responses, and high PD-1 expression is considered desirable in RA. However, the exact effects of the PD-1 signaling axis in RA remains to be elucidated.
Extracellular Galectin-3 (Gal-3) is suggested to be associated with RA disease severity and inflammation. In contrast, Gal-3 has immune suppressing capabilities in cancer. Thus, effects of Gal-3 are complex, but could potentially be explained by Gal-3s diverse effect on immune checkpoint receptors.
Here, we aimed to investigate whether Gal-3 is a binding partner to PD-1, and how this interaction affects PD-1 signaling as well as inflammatory markers.
Methods: Patient material consisted of synovial fluid (SF, n=25), plasma, peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) from chronic (c) RA patients. Patients presented with a disease flare when samples were obtained. Surface Plasmon Resonance (SPR) was performed using recombinant human (rh) Gal-3 and PD-1 to investigate potential binding. PD-1 and Gal-3 interaction in patient samples was measured by ELISA. Surface expression of Gal-3 and PD-1 on PBMCs and SFMCs, as well as intracellular IL-2 levels upon stimulation were assessed by flow cytometry. Statistical analysis and graphs were performed using GraphPad Prism 9.0.2 Software.
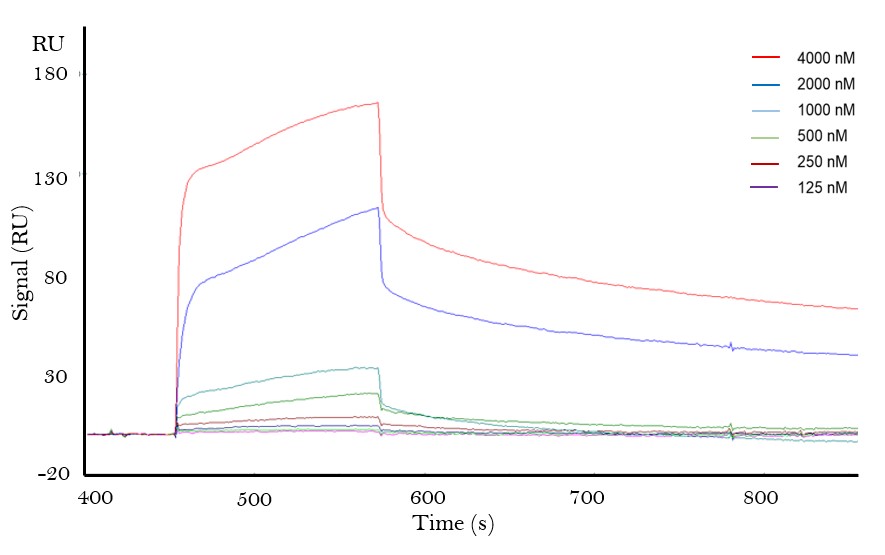
Results: Using SPR, we demonstrated that Gal-3 binds PD-1 as well as PD-L1 with an estimated KD of 10-6 and 10-5, respectively (Fig. 1). This binding was confirmed in SF and plasma samples from RA patients, where rhGal-3 could capture soluble PD-1 (Fig. 2). Significantly higher levels of PD-1 were detected by rhGal-3 in SF (n=25) compared to plasma samples from both RA (n=7, p=0.002) and healthy controls (n=7, p=0.001).
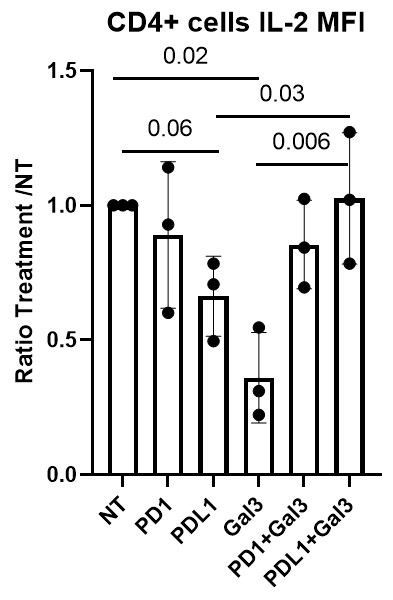
RA PBMCs (n=6) and SFMCs (n=6) were stimulated using anti-CD3/anti-CD28 stimulation . Significantly higher levels of cells expressing either PD-1 (p=0.007), as well cells positive for both PD-1 and Gal-3 (p=0.04), were observed in SFMCs compared to PBMCs, supporting the effects of Gal-3 in the local inflamed microenvironment. Treating stimulated CD4+ cells with rhPD–L1, caused IL-2 levels to decrease (p=0.059). When rhGal-3 was administered alone it also resulted in decreased IL-2 levels (p=0.02), however, treating stimulated CD4+ cells with both rhPD-L1 and rhGal-3 the effect of each treatment was repelled (p=0.03), which could be explained by Gal-3s capability to interact directly with PD-1 and its ligands (Fig. 3).
Conclusion: Here we showed how Gal-3 binds PD-1. Data support that the effect of Gal-3 on PD-1 signaling are more pronounced in a local inflammatory milieu in the synovium. Finally, when both Gal-3 and PDL1 were present, the anti-inflammatory effect of PD-1 signaling in decreasing IL-2 was repelled by high levels of local Gal-3.
To cite this abstract in AMA style:
Pedersen K, Nielsen M, Hvid M, Deleuran B, Greisen S. The Modulatory Capacity of Galectin-3 on the Programmed Death-1 Signaling Axis in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-modulatory-capacity-of-galectin-3-on-the-programmed-death-1-signaling-axis-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-modulatory-capacity-of-galectin-3-on-the-programmed-death-1-signaling-axis-in-rheumatoid-arthritis/