Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: CCN1, a matricellular protein with angiogenic and immunomodulatory properties, is overexpressed in endothelial and synovial tissues of rheumatoid arthritis (RA) patients. Previous findings demonstrated that CCN1 inhibition reduces synovial angiogenesis and structural damages in preclinical arthritis models. However, it remains unclear whether these therapeutic effects of CCN1 inhibition are mediated through a reduction in angiogenesis. This study investigates the ability of Cilengitide, a cyclic pentapeptide integrin inhibitor, to block CCN1-mediated effects on endothelial cells (ECs) and evaluates its potential therapeutic impact in the TNF-dependent Tg197 arthritis model.
Methods: The effects of CCN1 inhibition on angiogenesis through Cilengitide were assessed in vitro using ECs derived from circulating progenitors. Cells were cultured with CCN1 (0.2 µg/ml), either alone or with Cilengitide (10 µM). We analyzed capillary-like tube formation on Matrigel and EC migration using scratch assay. For in vivo studies, TNF-transgenic mice (Tg197) received intraperitoneal injections of either Cilengitide (10 mg/kg) or PBS three times weekly. Clinical arthritis scores were monitored bi-weekly, and after six weeks, paws were analyzed using micro-CT and histology. Immune profiling from the spleen of TG197 mice was performed via flow cytometry.
Results: In vitro, CCN1 induced capillary-like tube formation and EC migration / invasion, which were significantly inhibited by Cilengitide at a dose of 10µM. (Figure 1A-B), strongly supporting that CCN1 effects on ECs are mediated through integrins αvβ3 and αvβ5, which are critical for angiogenesis.In vivo, Cilengitide significantly improved clinical arthritis scores (Figure 1C). The AUC of the clinical score of Cilengitide-injected mice decreased significantly by 55±6% (p=0.020). Micro-CT analysis demonstrated better-preserved joint integrity in Cilengitide-treated mice, with markedly fewer bone erosions compared to controls (Figure 1D-E). Histological analysis of hind paw sections confirmed previous results, with significantly less inflammation and structural damage in Cilengitide-treated mice (Figure 1F-G).Flow cytometry showed decreased activation of CD4+ T cells in treated mice, with reduced PD-1 expression, indicating lower exhaustion levels following Cilengitide treatment. CD4+ T cells exhibited a shift in memory subsets, with fewer effector memory T cells and an increase in central memory and naïve T cells (Figure 1H).
Conclusion: Cilengitide effectively inhibits CCN1-induced angiogenesis in vitro, confirming that CCN1 exerts its effects on endothelial cells through integrins αvβ3 and αvβ5. In vivo, Cilengitide significantly reduces clinical, structural, and histological damage in the Tg197 arthritis model while modulating immune profiles. These findings suggest that CCN1’s effects in RA, including structural and inflammatory damage, are mediated by its role in promoting pathological angiogenesis. Targeting CCN1 and associated pathways offers a promising therapeutic strategy for RA, complementing current treatments.
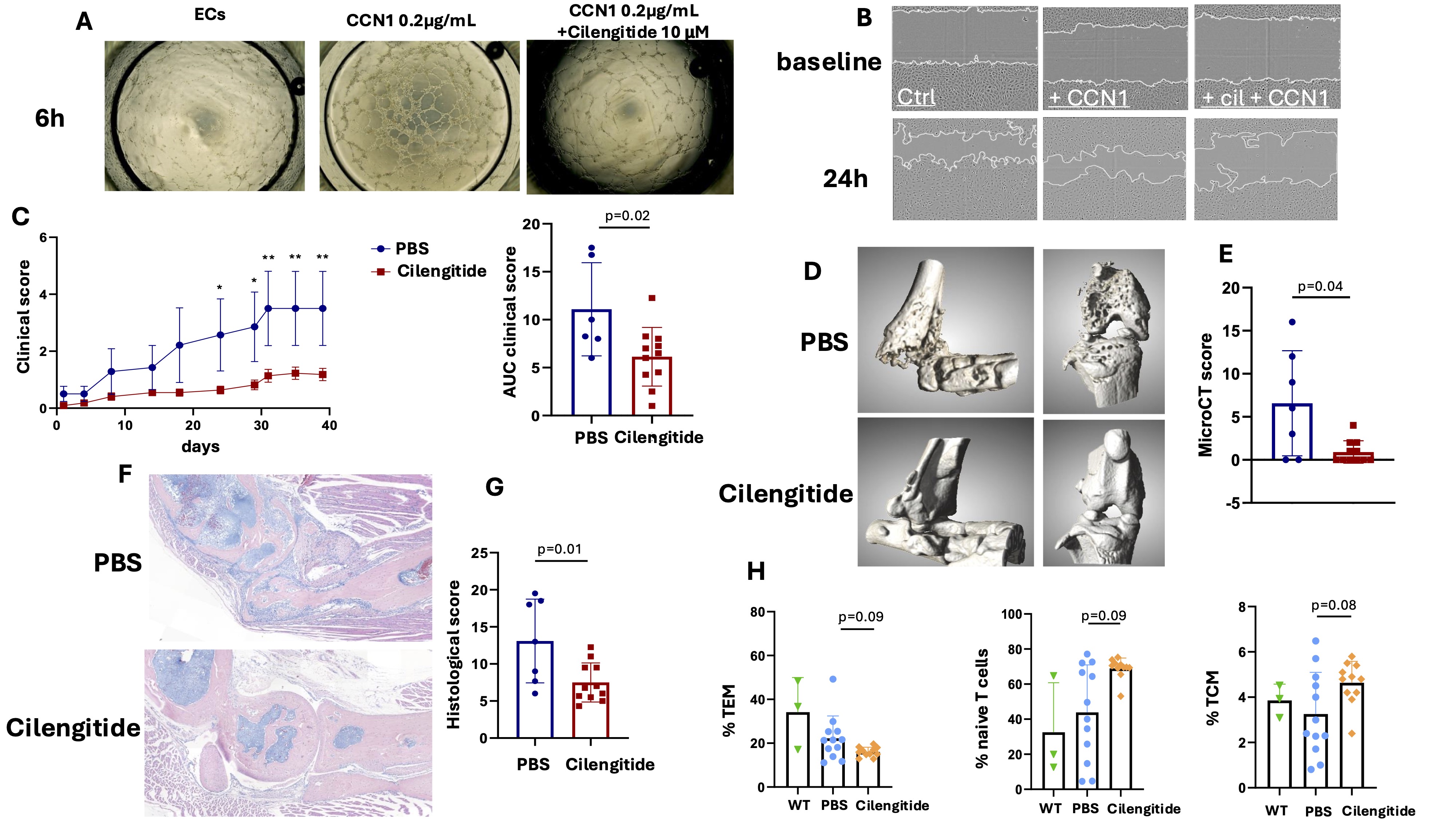 A, Representative images of tube formation at 6 hours in RA endothelial cells (ECs) alone, or following treatment with recombinant CCN1 (0.2 g/mL) with or without Cilengitide (10 M) (4x magnification). B, Representative images of EC migration at baseline and 24 hours in RA ECs alone, or following treatment with recombinant CCN1 (0.2 g/mL) with or without Cilengitide (10 M).
A, Representative images of tube formation at 6 hours in RA endothelial cells (ECs) alone, or following treatment with recombinant CCN1 (0.2 g/mL) with or without Cilengitide (10 M) (4x magnification). B, Representative images of EC migration at baseline and 24 hours in RA ECs alone, or following treatment with recombinant CCN1 (0.2 g/mL) with or without Cilengitide (10 M).
C, Experimental arthritis in Tg197 mice receiving IP injections of Cilengitide (10 mg/kg) or PBS (100 L) 3 times weekly. Y-axis shows clinical score and the area under the curve (AUC) of the clinical score (PBS: n=7 mice, Cilengitide, n=11 mice). D, Representative 3D reconstructions of ankles, tarsus and knees of Tg197 mice treated with Cilengitide or PBS. E, Y-axis shows the mean erosion score (from 0 to 16). F, Sections of ankle and tarsus joints from Tg197 mice receiving Cilengitide or PBS stained with hematoxylin (scale bar = 200m). G, Histomorphometric analysis of the area of synovitis and bone destruction. H, Flow cytometry analysis of T cell subsets (effector memory T cells, TEM, naive T cells and central memory T cells, TCM) from the spleen of wild type (WT) mice (n=3) and Tg197 mice treated with Cilengitide or PBS. All data are shown as the means ± SD. Statistical tests: Student’s t test for 2-group comparisons, ANOVA with Dunnett’s multiple comparisons test for 3-group comparisons. Data are representative of a single experiment.
To cite this abstract in AMA style:
Avouac J, lesturgie m, Gonzalez V, Arulananthan S, Cauvet A, Tilotta F, Allanore Y. The Integrin Inhibitor Cilengitide Targets CCN1-Mediated Angiogenesis and Reduces Disease Severity in a Preclinical Rheumatoid Arthritis Model [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-integrin-inhibitor-cilengitide-targets-ccn1-mediated-angiogenesis-and-reduces-disease-severity-in-a-preclinical-rheumatoid-arthritis-model/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-integrin-inhibitor-cilengitide-targets-ccn1-mediated-angiogenesis-and-reduces-disease-severity-in-a-preclinical-rheumatoid-arthritis-model/
