Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: In rheumatoid arthritis (RA), chronic synovial inflammation is accompanied by fibrotic responses that together lead to pannus formation and progressive joint damage. Exposure to malondialdehyde-acetaldehyde adducts (MAA) and/or citrullinated (CIT) proteins in isolation or in combination significantly influence in vitro inflammatory and fibrotic responses in macrophage and fibroblast cell lines in isolation. However, the potential interaction between these two cell types in the context of exposure to different post-translationally (co-)modified proteins has not been well delineated. The purpose of this study was to evaluate whether soluble factors secreted by macrophages (U937 cells) in response to MAA and/or CIT modified proteins affect the inflammatory and/or fibrotic responses by human fibroblast-like synoviocytes derived from RA synovium (HFLS-RA).
Methods: PMA-treated U937 cells were stimulated with MAA, CIT, or MAA-CIT modified human serum albumin (HSA) or fibrinogen (FIB) for 24 hours. Cell supernatants were collected and analyzed for the following cytokines: MIP-1α, IL-1β, and IL-18 using Meso Scale Discovery (MSD) platform. In a separate experiment, HFLS-RA cells were co-incubated with previously collected U937 supernatants (following antigen stimulation as above) and mRNA was isolated to evaluate fibrotic markers with PCR: type II collagen (COL2A1), MMP9, and vimentin (VIM). To account for the fact that increased mRNA expression could derive from modified antigens in the supernatant, we set a control following direct stimulation of HFLS-RA cells with MAA and/or CIT modified antigen in the absence of supernatants. One-way ANOVA was used to compare cytokine and mRNA levels across groups.
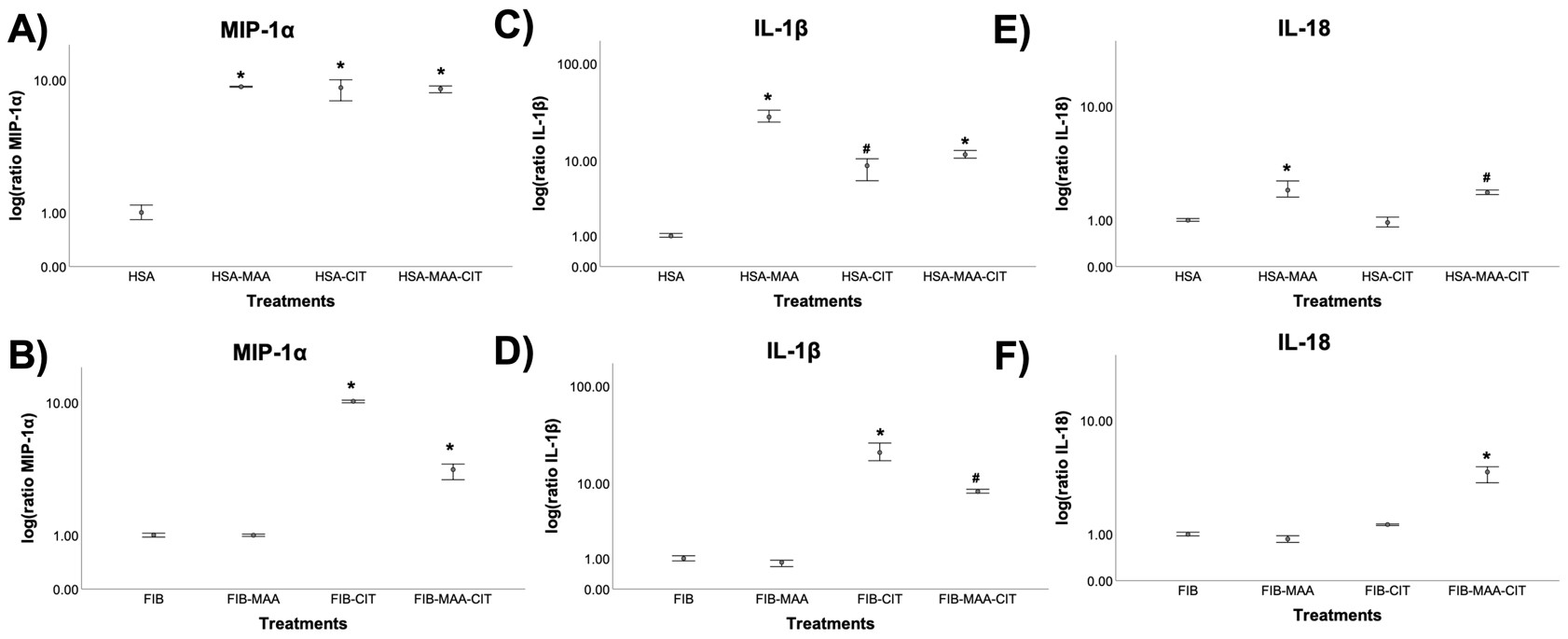
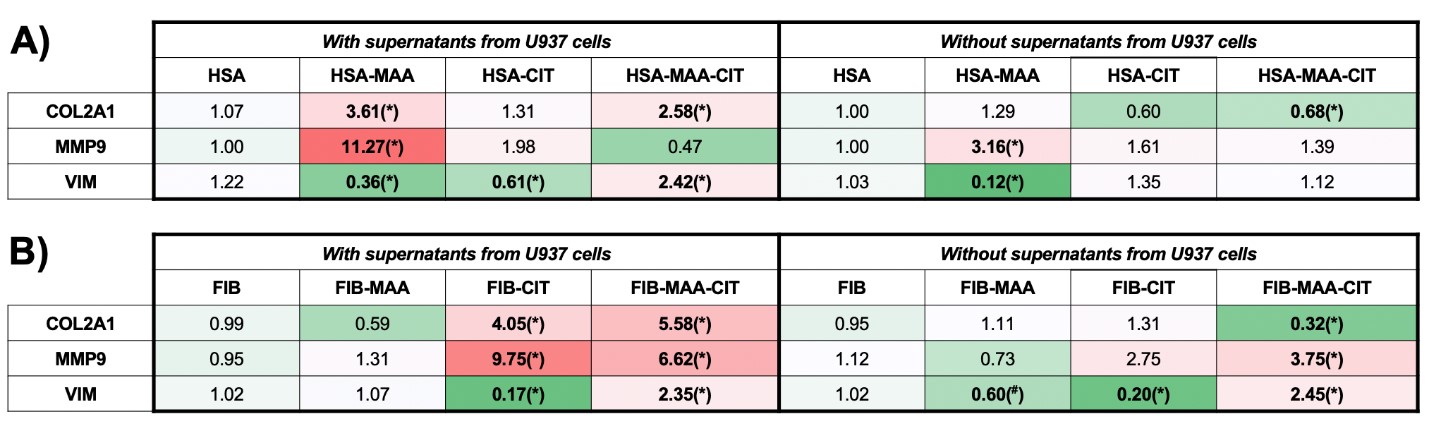
Results: Stimulation of U937 cells with MAA, CIT, or MAA-CIT modified HSA demonstrated a significant increase in MIP-1α and IL-1β release (Fig.1A, C). In contrast, MIP-1α and IL-1β release were the greatest with FIB-CIT stimulation, followed by FIB-MAA-CIT (Fig.1B, D). IL-18 concentrations were increased significantly with HSA-MAA, HSA-MAA-CIT, and FIB-MAA-CIT stimulation (Fig.1E, F). When HFLS-RA cells were stimulated with HSA-MAA treated U937 supernatants, mRNA levels of COL2A1 and MMP9 increased 3 and 4-fold, respectively, compared to HSA-MAA stimulation without U937 supernatants (Fig.2A). Dually modified (MAA-CIT) HSA and FIB treated U937 supernatants increased HFLS-RA mRNA levels for VIM (Fig.2A, B). FIB-CIT and FIB-MAA-CIT treated U937 supernatants increased HFLS-RA mRNA levels for both COL2A1 and MMP9 as compared to direct stimulation of HFLS-RA with FIB-CIT and FIB-MAA-CIT antigens without U937 supernatants (Fig.2B).
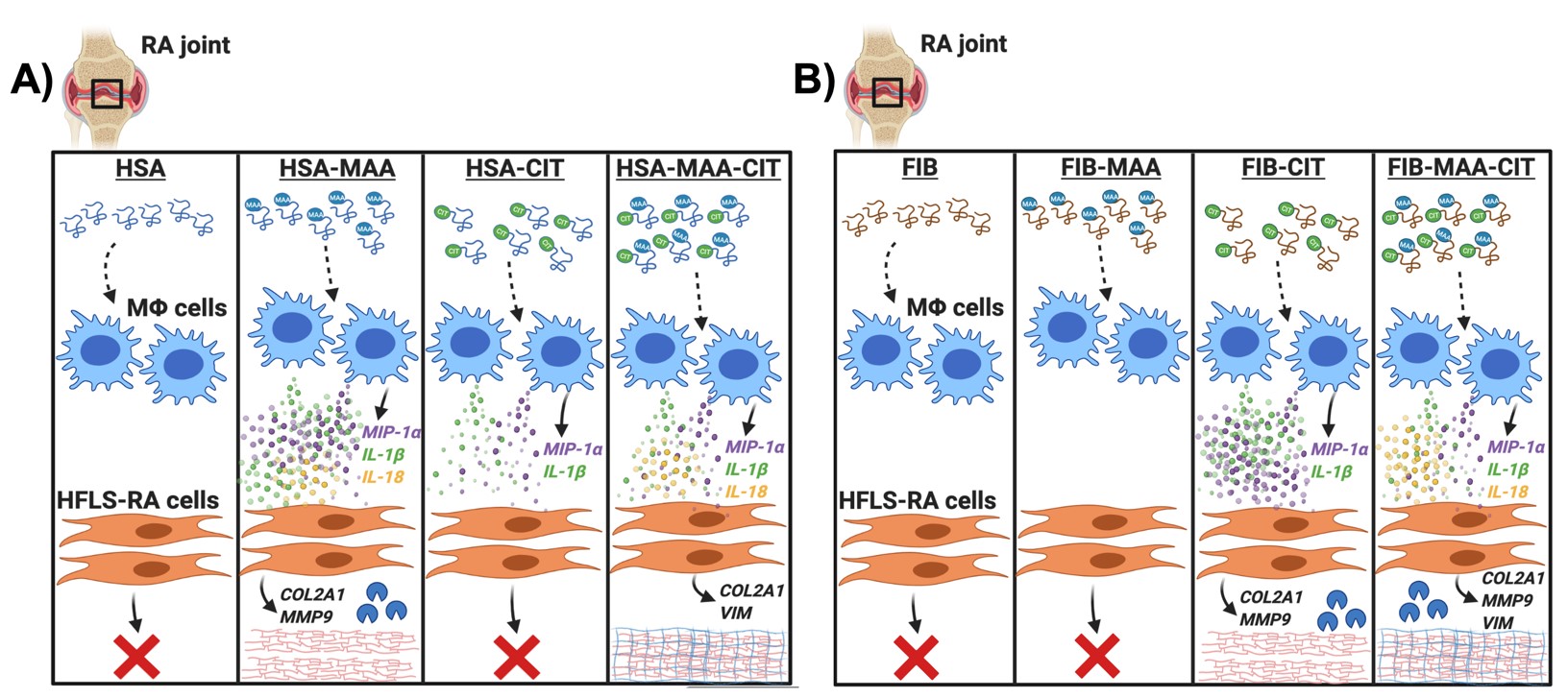
Conclusion: These results suggest a disease model whereby MAA and/or CIT modified proteins stimulate macrophages to release a unique combination of soluble mediators (such as MIP-1α, IL-1β or IL-18) that activate HFLSs and upregulate protein expression driving tissue fibrosis, in an antigen-specific manner (Fig. 3). Together with prior studies demonstrating MAA and CIT co-localization in RA synovium, these results indicate that MAA and/or CIT modified antigens may play a direct role in the development of fibrosis that has been observed in RA joint pathogenesis.
To cite this abstract in AMA style:
Aripova N, Duryee M, Ryan E, Maloley P, England B, O'Dell J, Mikuls T, Thiele G. The Impact of Macrophages Stimulated with Malondialdehyde-Acetaldehyde And/or Citrulline Modified Proteins on Fibroblasts Activation [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-macrophages-stimulated-with-malondialdehyde-acetaldehyde-and-or-citrulline-modified-proteins-on-fibroblasts-activation/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-macrophages-stimulated-with-malondialdehyde-acetaldehyde-and-or-citrulline-modified-proteins-on-fibroblasts-activation/