Session Information
Date: Sunday, November 7, 2021
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Miscellaneous Aspects of RA (0786–0812)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Comorbid conditions have been shown to negatively influence the achievement of treatment targets in rheumatoid arthritis (RA) patients. The comorbid conditions may bias the subjective measures of the widely used indices for evaluation of disease activity. The objective is to look at the relationships between comorbidities and components of common clinical disease activity scores, such as tender (TJC) and swollen (SJC) joint count, patient global assessment (PtGA), physician global assessment (MDGA), C-reactive protein (CRP) and pain in early RA.
Methods: Using a validated tool for the assessment of comorbidity burden specifically developed for rheumatology, the Rheumatic Disease Comorbidity Index (RDCI), the influence of comorbidities on each component’s trajectory in time has been assessed in early rheumatoid arthritis (ERA) patients over the first year of treatment with conventional synthetic DMARDS using data from the Canadian Early Arthritis Cohort (CATCH). The adjusted effects of RDCI scores (0, 1, 2, and ≥ 3) on the trajectory of the SDAI, on each component of the SDAI, and on pain (visual analog scale) was evaluated over the first year of follow-up with generalized estimating equations (GEE). Data were adjusted for possible confounders including baseline sociodemographic factors, disease activity and treatments.
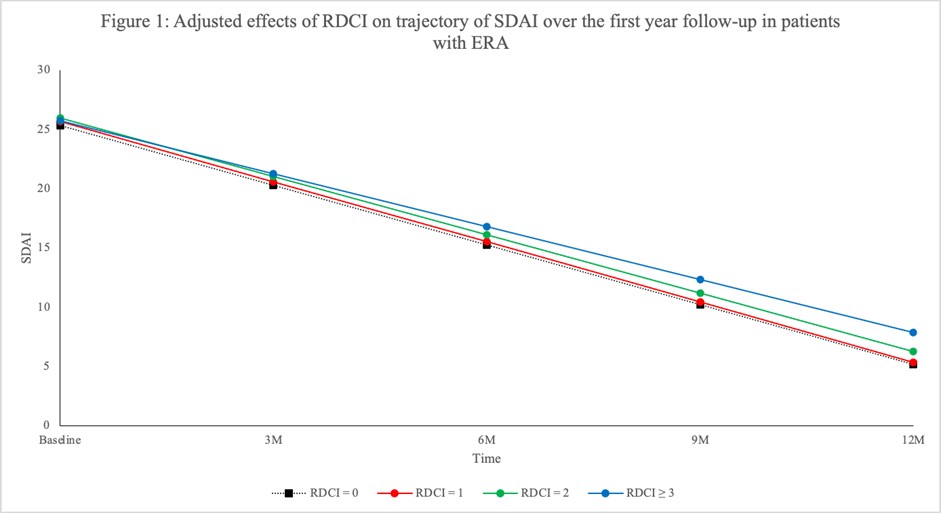
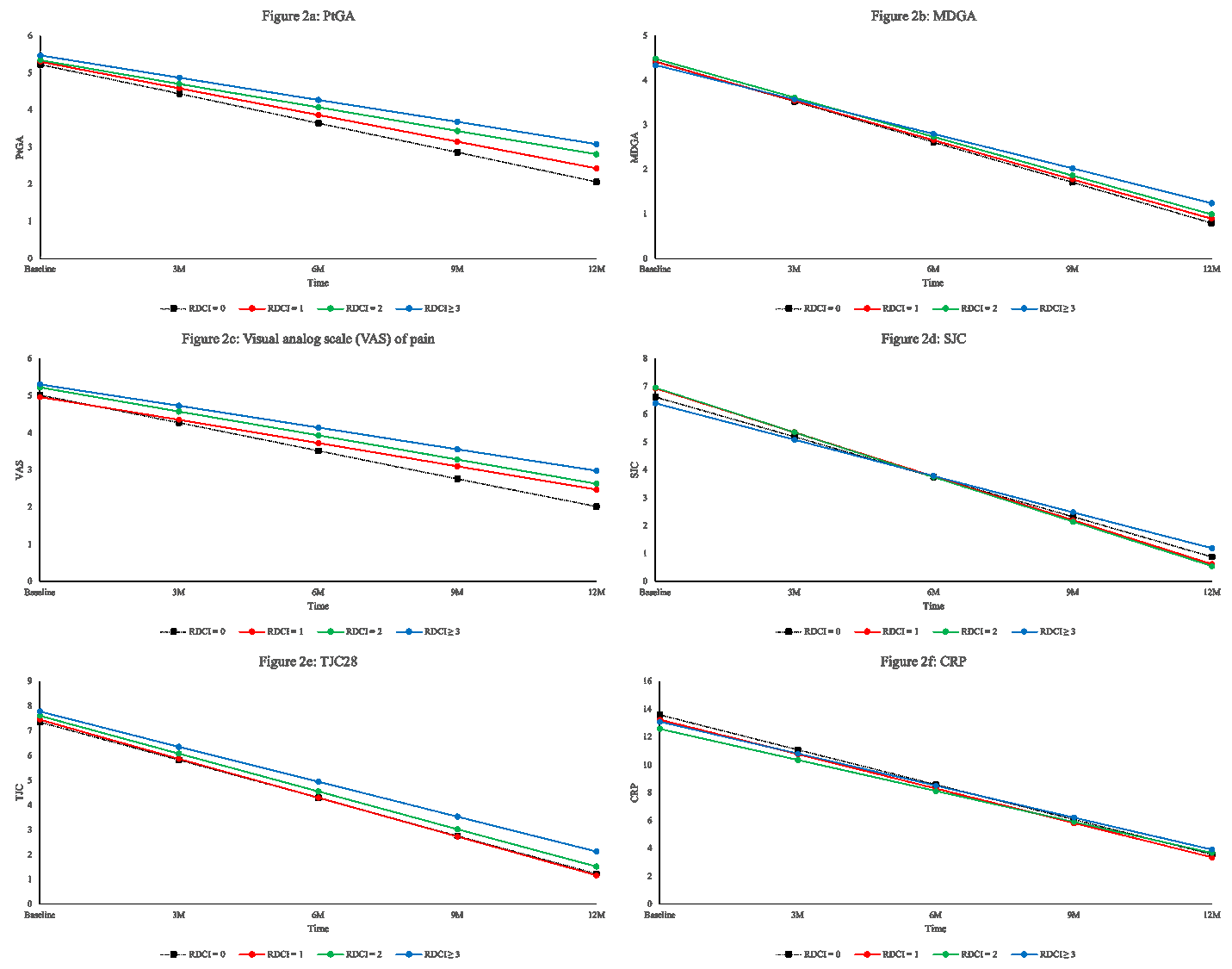
Results: This sample size included 2248 ERA patients with a mean symptom duration (SD) of 5.71 (2.96) months; mean age (SD) was 55 (15) years old and 72% were female. At baseline, 1664 (74%) were treated with methotrexate with a mean weekly dose (SD) of 20.0 mg (4.2) and 1340 (60%) also received other conventional synthetic DMARDs. The mean (SD) SDAI at enrolment was 29 (15) and 90% were classified as having moderate-high SDAI. RDCI scores of 0, 1, 2 or ³3 were obtained in 888 (40%), 547 (24%), 451 (20%), and 362 (16%) participants, respectively. Although disease activity did not differ by comorbidity status at baseline, patients with RDCI of 0 had better improvement (rate of change) in SDAI (Figure 1), PtGA, MDGA and pain (Figure 2a,b,c) over time relative to patients with multiple RDCI conditions (p< 0.05). A significant higher rate of change in SJC was observed in patients with RDCI of 1 and 2 compared with participants having higher RDCI score (p= 0.01) (Figure 2d). The RDCI scores were not significantly associated with the change of TJC and CRP (Figure 2e and f) over one year.
Conclusion: In this ERA cohort, having multiple comorbidities was associated with worse improvement and disease activity assessed by SDAI over the first year of treatment. SJC, PtGA, and MDGA were the components of SDAI that were most influenced by the presence of comorbidities. The results demonstrate a negative effect of having comorbidities at disease onset of RA on the evolution of both patients and physicians reported outcomes.
To cite this abstract in AMA style:
Chen L, Schieir O, Valois M, Pope J, Bartlett S, Boire G, Hazlewood G, Hitchon C, Keystone E, Tin D, Thorne C, Singbo N, Bykerk V, Bessette L. The Impact of Comorbidities on the Simple Disease Activity Index (SDAI) and Its Components over the First Year of Follow-up – an Analysis from the Canadian Early Arthritis Cohort (CATCH) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-comorbidities-on-the-simple-disease-activity-index-sdai-and-its-components-over-the-first-year-of-follow-up-an-analysis-from-the-canadian-early-arthritis-cohort-catch/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-comorbidities-on-the-simple-disease-activity-index-sdai-and-its-components-over-the-first-year-of-follow-up-an-analysis-from-the-canadian-early-arthritis-cohort-catch/