Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Metabolic & Crystal Arthropathies – Basic & Clinical Science (2585–2590)
Session Type: Abstract Session
Session Time: 1:00PM-1:15PM
Background/Purpose: Colchicine is recommended for the treatment and prophylaxis of gout flares and approved for secondary prevention of ischemic cardiovascular disease (CVD). While its primary anti-inflammatory mechanism is thought to be tubulin disruption, other biological pathways are likely impacted. This study evaluated the effect of prophylactic colchicine on gene expression among individuals with gout enrolled in the 72-week STOP Gout clinical trial (O’Dell JR et al. NEJM Evid 2022).
Methods: STOP Gout participants (Nf940) met classification criteria for gout, were randomized to treat-to-target allopurinol or febuxostat, and received anti-inflammatory prophylaxis (90% colchicine). Prophylaxis was stopped at 24 weeks but could be continued or resumed up to week 48 if desired or clinically indicated. This study limited to those with whole blood RNA PaxGene (BD Biosciences) tube samples at 24 and/or 48 weeks (N = 259). Those identified as in active flare or with 0-10 pain score >3 at the time of lab draw were excluded (N = 45). Sequencing libraries were prepared from extracted RNA using Illumina Stranded Total RNA Prep with Ribo-Zero™ Plus rRNA Depletion + Globin Reduction. Sequence reads were aligned and counted via STAR aligner software. Normalized counts were generated using DESeq2. Differential expression was evaluated using mixed effects negative binomial regression with subject-specific random intercepts accounting for repeated measures in the GLMMseq package (R 4.4.1). For each gene, hypothesis tests of fixed effects were made for colchicine use status, timepoint, and colchicine:time interaction. Genes were filtered for false discovery rate-adjusted p-value < 0.1, |log2 fold change| >0.2, and absence of time interaction.
Results: Of 214 gout participants included, 165 had samples at one timepoint and 49 at both (total 263 samples). At 24 weeks, 98 of 132 samples (74.2%) were on colchicine, whereas 33 of 131 (25.1%) were on colchicine at 48 weeks. There were 29 genes associated with colchicine use (Figure 1; 16 under-expressed and 13 overexpressed). Nineteen had a described physiologic function on NIH Gene and literature review and were descriptively classified, most commonly as related to innate immunity (Table 1). Delta hemoglobin (HBD) was suppressed on colchicine, consistent with its known potential for anemia. Long non-coding RNA LINC02470, previously described as a correlate of transforming growth factor (TGF)-β-mediated inhibition of the innate immune response, was the most overexpressed gene on colchicine. SLC2A5, a requisite gene in dietary fructose-induced hypertension, and TNNT1, a troponin subunit that regulates myocardial contraction, were under-expressed on colchicine.
Conclusion: This study identifies potential mechanisms underlying the systemic impact of colchicine in individuals with gout. The most overexpressed gene, LINC02470, is a novel finding in gout but has been shown to correlate with innate immune inhibition and is also in close proximity to CLEC12A, which is known to bind monosodium urate crystals and suppress immune response. SLC2A5 is essential for fructose-induced hypertension, and its reduced expression with colchicine suggests one potential link to CVD risk reduction.
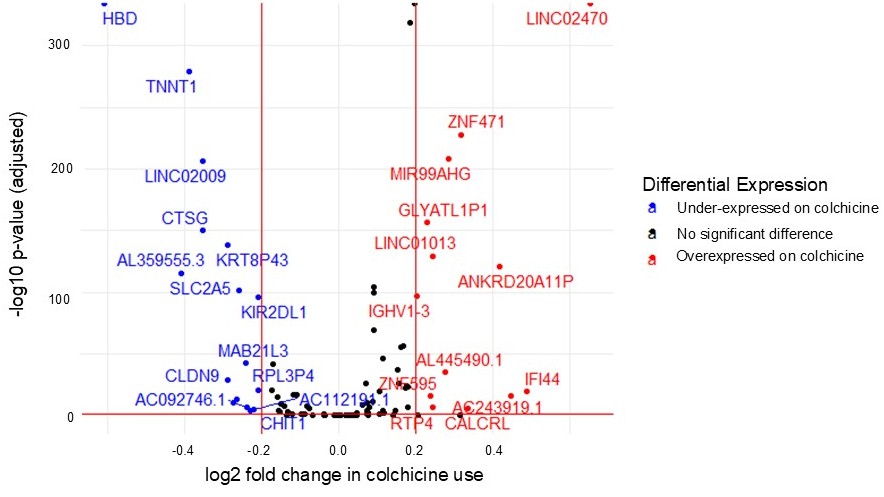 Figure 1. Gene expression differences by colchicine use among STOP Gout participants at 24 and 48 weeks.
Figure 1. Gene expression differences by colchicine use among STOP Gout participants at 24 and 48 weeks.
Cut-offs for significance defined as absolute log2 fold change >0.2 and adjusted p-value < 0.1.
.jpg) Table 1. Description of genes differentially expressed on colchicine with functional classification.
Table 1. Description of genes differentially expressed on colchicine with functional classification.
Abbreviations: fold change (FC)
Negative log2 fold change (grey) is under-expressed on colchicine. Positive log2 fold change (orange) is overexpressed on colchicine. Functional classification of “-“ denotes none established.
To cite this abstract in AMA style:
Wheeler A, Lu G, Vazquez A, Edberg J, Gaffo A, Johnson T, Duryee M, O'Dell J, Newcomb J, Pillinger M, Terkeltaub R, Ferguson R, Brophy M, Neogi T, England B, Mikuls T, Merriman T, Reynolds R. The effect of prophylactic colchicine use on gene expression in gout [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-effect-of-prophylactic-colchicine-use-on-gene-expression-in-gout/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-prophylactic-colchicine-use-on-gene-expression-in-gout/
