Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Progression of interstitial lung disease (ILD) reduces long-term survival in patients with systemic sclerosis (SSc). Monitoring with lung function testing every 3–6 months for the 1st year and less frequently afterwards is recommended in the recent ACR/Chest guidelines. Follow-up HRCT is recommended as needed. The objective of this study was to assess the impact of serial HRCT to identify ILD progression and its ability to predict mortality.
Methods: We included all SSc-ILD patients from Oslo and Zurich with ILD on HRCT, and available consecutive annual lung function assessments including forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO), HRCT, respiratory symptoms, and vital status at study end. ILD progression was defined by the 2022 ATS/ERS/JRS/ALAT guideline progressive pulmonary fibrosis (PPF) criteria over 12 ±3 months. The PPF criteria are consisting of the following with the subcriteria (1) worsening of respiratory symptoms; (2) absolute decline in FVC ≥5% or in DLCO ≥10% and (3) disease progression on HRCT. HRCTs were assessed by an experienced radiologist. We assessed the prevalence of PPF and the subcriteria comprising the definition. Cox regression was applied to identify the impact of PPF and its subcriteria on mortality. Kaplan Meier estimates were used to analyze 3-year survival.
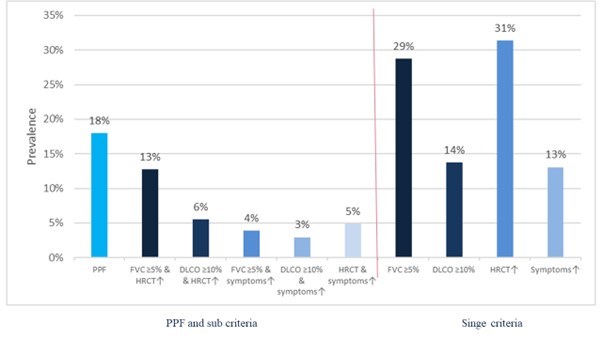
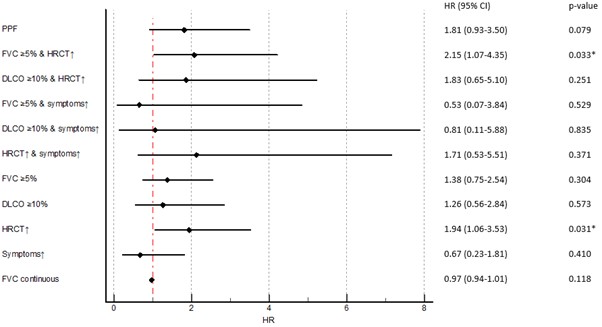
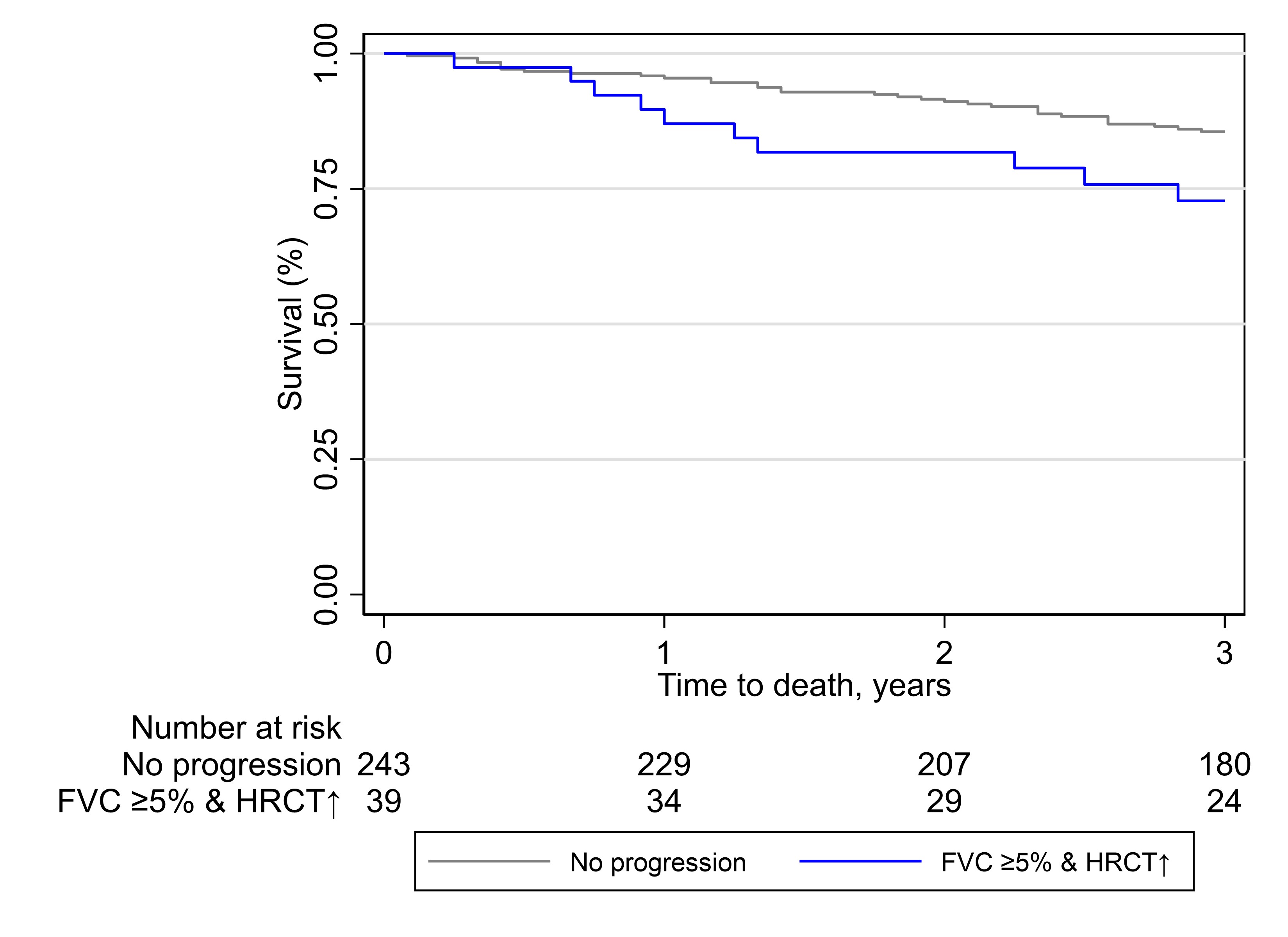
Results: We identified 306 SSc-ILD patients mirroring a typical SSc-ILD population with 74 (24%) males, 120 (39%) with diffuse SSc, 114 (37%) with anti-topoisomerase I antibodies, mean disease duration of 9.3 (±11.3) years, mean FVC of 87% (±19.8), DLCO of 65% (±19.6) and 81 (28%) with >20% of fibrosis on HRCT. We identified 55 (18%) SSc-ILD patients with PPF over 12 ±3 months. Fulfilment of the PPF criteria was mainly driven by the combination of absolute FVC decline ≥5% and worsening on HRCT (39 (13%)), followed by absolute DLCO decline ≥10% and worsening on HRCT (17 (6%)) and worsening on HRCT and respiratory symptoms (15 (5%)) (Figure 1). We assessed the impact of PPF and its subcategories on mortality and determined which specific criteria had the strongest impact. Over mean 3.5 (±0.87) years, 45 (15%) patients died, with 12 (27%) showing PPF over the first 12 months. While PPF itself was not associated with mortality, worsening on HRCT alone as well as in combination with FVC ≥5% decline predicted mortality (Figure 2). PPF showed no significant association with 3-year-mortality, but the combination of worsening on HRCT and absolute FVC decline ≥5% was significantly reducing the 1- and 3-year survival with p=0.039 (Figure 3).
Conclusion: Worsening on HRCT is the driving parameter of the PPF criteria and the strongest predictor for reduced survival. Annual HRCT is therefore of high important to identify ILD progression in SSc and to risk-stratify patients. Not using HRCT leads to missed opportunities for timely identification of ILD progression and for optimized disease management.
To cite this abstract in AMA style:
Hoffmann-Vold A, Beyeler S, Petelytska L, Fretheim H, Aaløkken T, Becker M, Jenssen Bjørkekjær H, Brunborgg C, Bruni C, Clarenbach C, Diep P, Dobrota R, Durheim M, Elhai M, Frauenfelder T, Jordan S, Langballe E, Midtvedt Ø, Mihai C, Sarbu A, Sprecher M, Molberg Ø, Distler O. The Critical Role of Annual HRCT in Identifying ILD Progression and Assessing Outcome in SSc [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-critical-role-of-annual-hrct-in-identifying-ild-progression-and-assessing-outcome-in-ssc/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-critical-role-of-annual-hrct-in-identifying-ild-progression-and-assessing-outcome-in-ssc/