Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Rheumatoid arthritis (RA) patients and TNF-transgenic mice have lymphatic dysfunction (1). Recently, we showed mast cells involvement, as genetic ablation and drug inhibition decreased lymphatic drainage and exacerbated arthritis in TNF-Tg mice (2). Single-cell RNA sequencing studies identified genes (i.e., Efhd1) selectively expressed in popliteal lymphatic vessels (PLVs) (3). However, the location and function of Efhd1–expressing cells and their relationship with mast cells are not known. Here we aimed to characterize a novel Efhd1-CreERT2 mouse model as a tool for PLV gain and loss-of-function studies and elucidate the mechanisms of peri-lymphatic mast cell regulation.
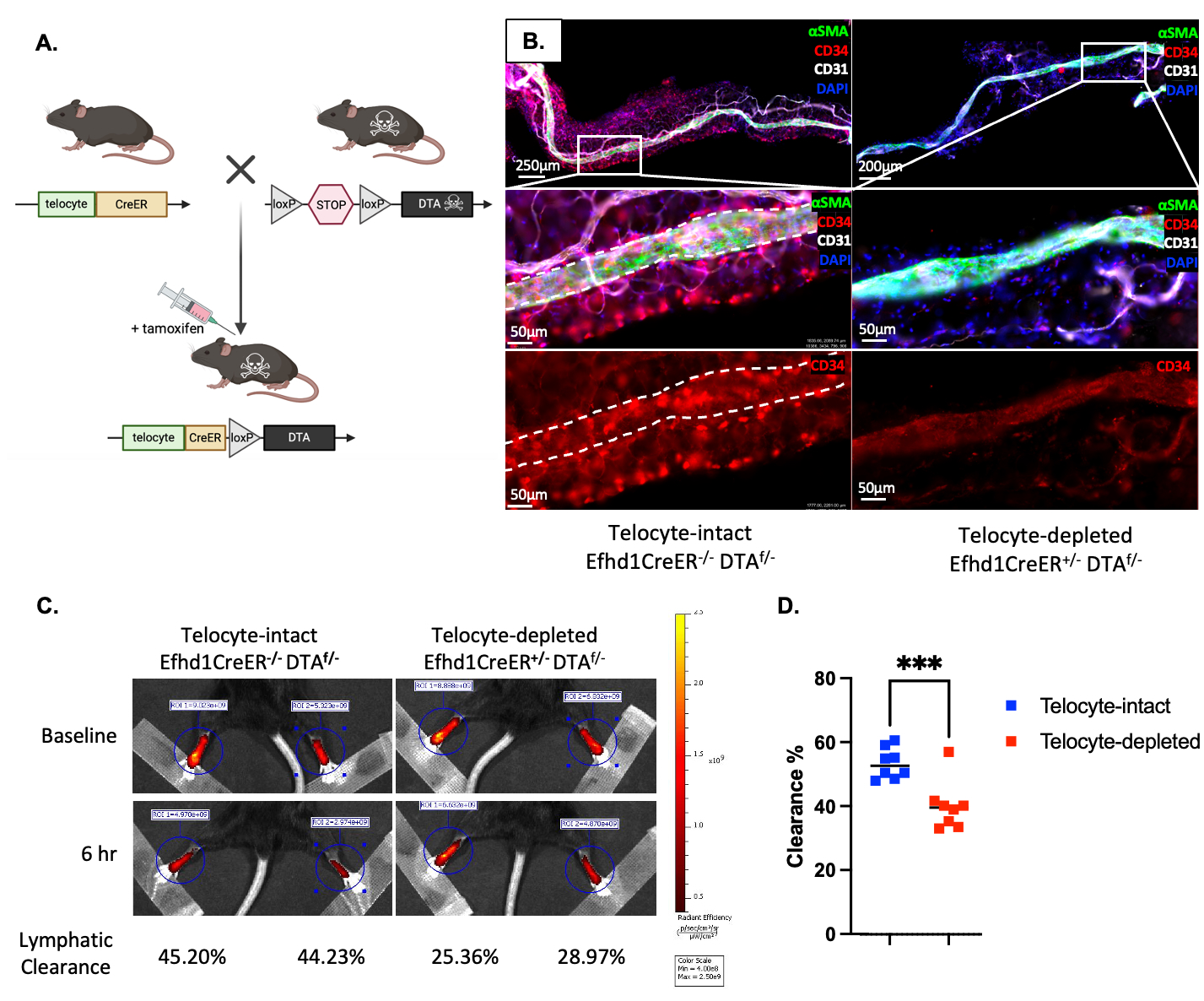
Methods: Efhd1-CreERT2 mice were crossed to Ai9-tdTomato (tdT) reporter mice. The heterozygous offspring were treated with tamoxifen as neonates or adults for lineage tracing studies in the popliteal vasculature and adjacent adipose tissue via immunofluorescent microscopy (IFM) with subsequent transmission electron microscopy (TEM). The Efhd1-CreERT2 mice were also crossed to ROSA-diphtheria toxin A (DTA) mice to determine in vivo cell depletion efficiency and lymphatic clearance by IFM and near-infrared imaging of indocyanine green (NIR-ICG) injected into the footpad, respectively.
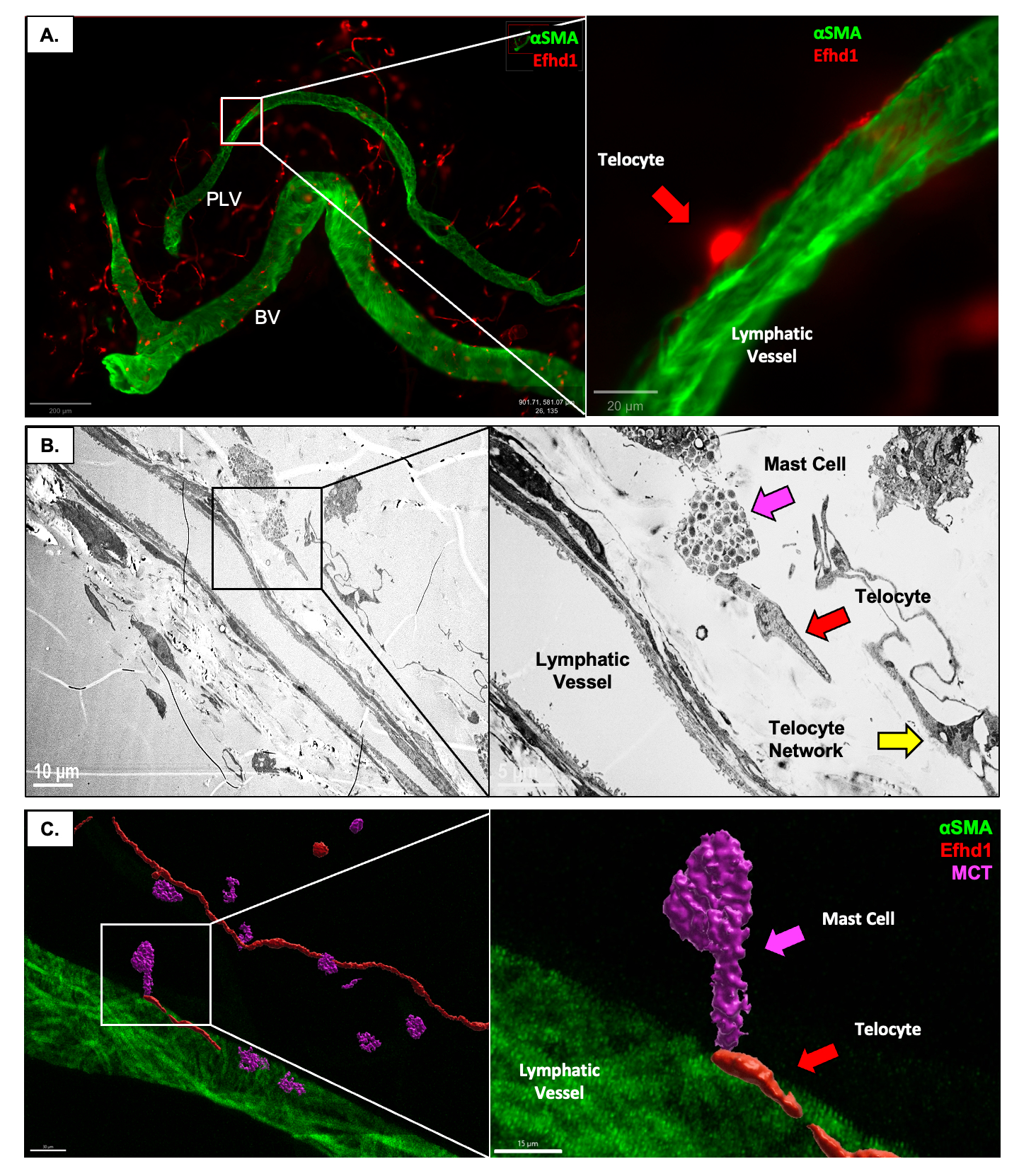
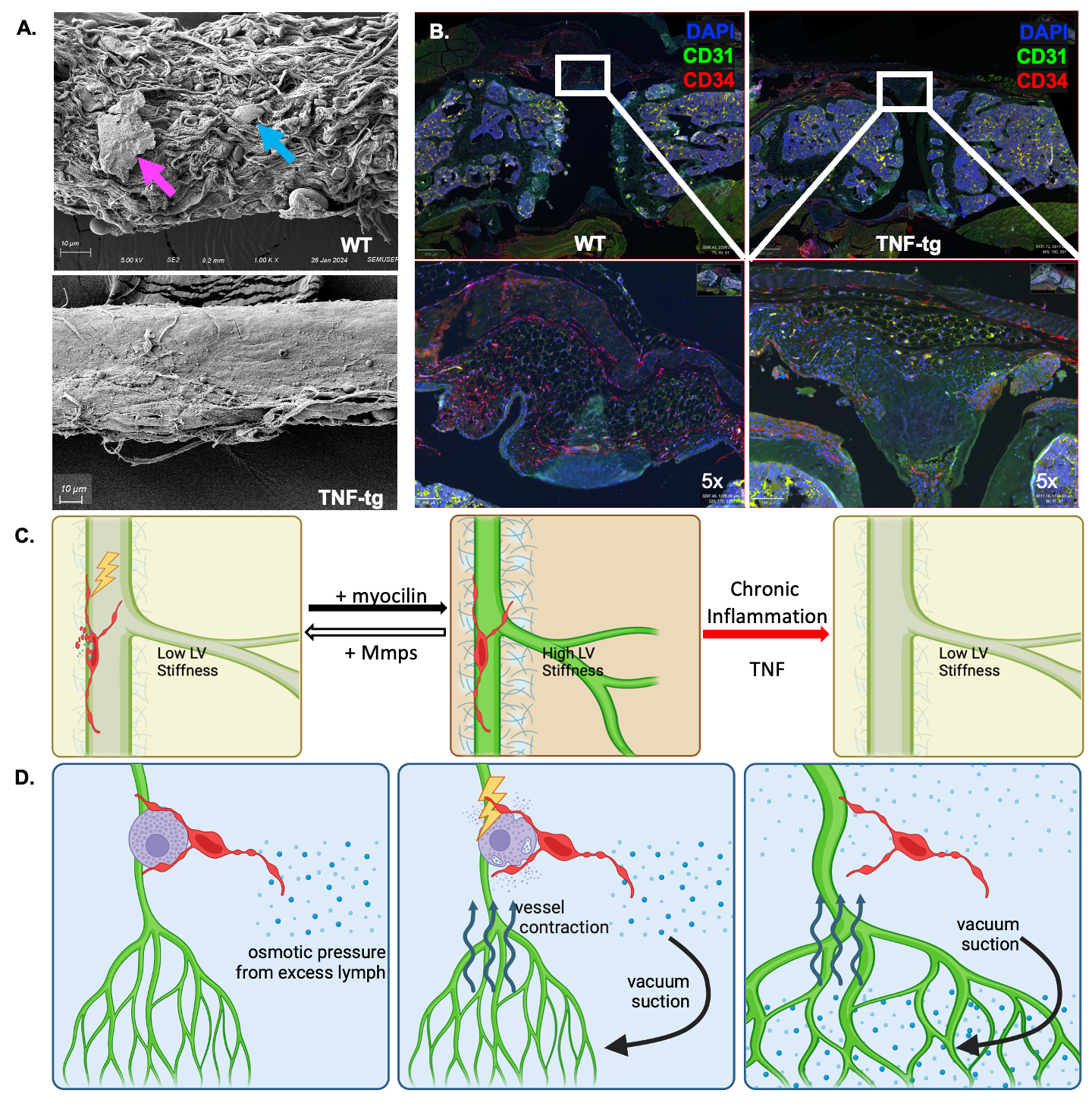
Results: Developmental and adult lineage tracing demonstrated Efhd1-tdT+ labeling of peri-vascular telocytes based on their fibroblastic morphology and characteristic telopods (Figure 1A). Telocyte identity was confirmed by CD31–/CD34+/Vimentin+ IFM (4), and histomorphometry revealed their proportion in WT synovium (84.15%±7.99%) was significantly decreased in TNF-Tg synovium (26.04%±7.38%; p< 0.001) (Figure 3B). Parallel TEM (Figure 1B) and IFM (Figure 1C) revealed tdT+ telopods integrated along the PLV, and within mast cells indicating direct cytoplasmic connections between these cells. DTA-induced depletion of telocytes resulted in reduced lymphatic clearance (Figure 2), suggesting that telocytes may mediate mast cell and PLV communication to regulate lymphatic function.
Conclusion: We developed and validated a novel Efhd1-CreERT2 transgenic mouse for inducible gain and loss-of-function studies in telocytes, whose function in joint homeostasis and arthritis is unknown. Two distinct subtypes of peri-PLV telocytes exist: one with telopods longitudinally attached along PLVs, and the other with telopods integrated within the plasma membrane of mast cells. Based on these findings, we hypothesize that: 1) PLV telocytes sense and modulate the extracellular matrix (ECM) stiffness of PLVs, potentially through the secretion of myocilin, TIMPs and MMPs (3) for ECM remodeling (Figure 3A&C); and 2) interstitial telocytes that may sense osmotic pressure and regulate PLV contractility, potentially by interacting with mast cells to induce the release of factors (e.g. histamine) that modulate PLV contractions (Figure 3D). Our findings are also consistent with the loss of telocytes in RA synovium (4), phenotypic changes to a yet to be defined population of fibroblastic cells in RA synovium (5) that may be telocytes, and lymphatic dysfunction during disease progression (1).
To cite this abstract in AMA style:
Peng Y, Kenney H, Bentley K, Xing L, Korman B, Ritchlin C, Schwarz E. Telocytes Integrated into Mast Cells and Joint-Draining Lymphatic Vessels Potentially Regulate Lymphatic Clearance [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/telocytes-integrated-into-mast-cells-and-joint-draining-lymphatic-vessels-potentially-regulate-lymphatic-clearance/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/telocytes-integrated-into-mast-cells-and-joint-draining-lymphatic-vessels-potentially-regulate-lymphatic-clearance/