Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: T Cell Biology & Targets in Autoimmune & Inflammatory Disease
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Fibroblast activation protein alpha (FAPa) expressing fibroblasts orchestrate tissue inflammation and damage in rheumatoid arthritis (RA) as well as tissue immunity in primary Sjögren’s syndrome (PSS) through the formation of tertiary lymphoid structures (TLS; Croft et al., Nature 2019 & Nayar et al., PNAS 2019). As a result, therapeutic targeting of these resident cells has the potential to reset the inflammatory tissue microenvironment, in favour of resolution. Our aim was to determine the therapeutic efficacy of FAPa-targeted chimeric T (FAPCAR-T) cell immunotherapy in inflammatory disease.
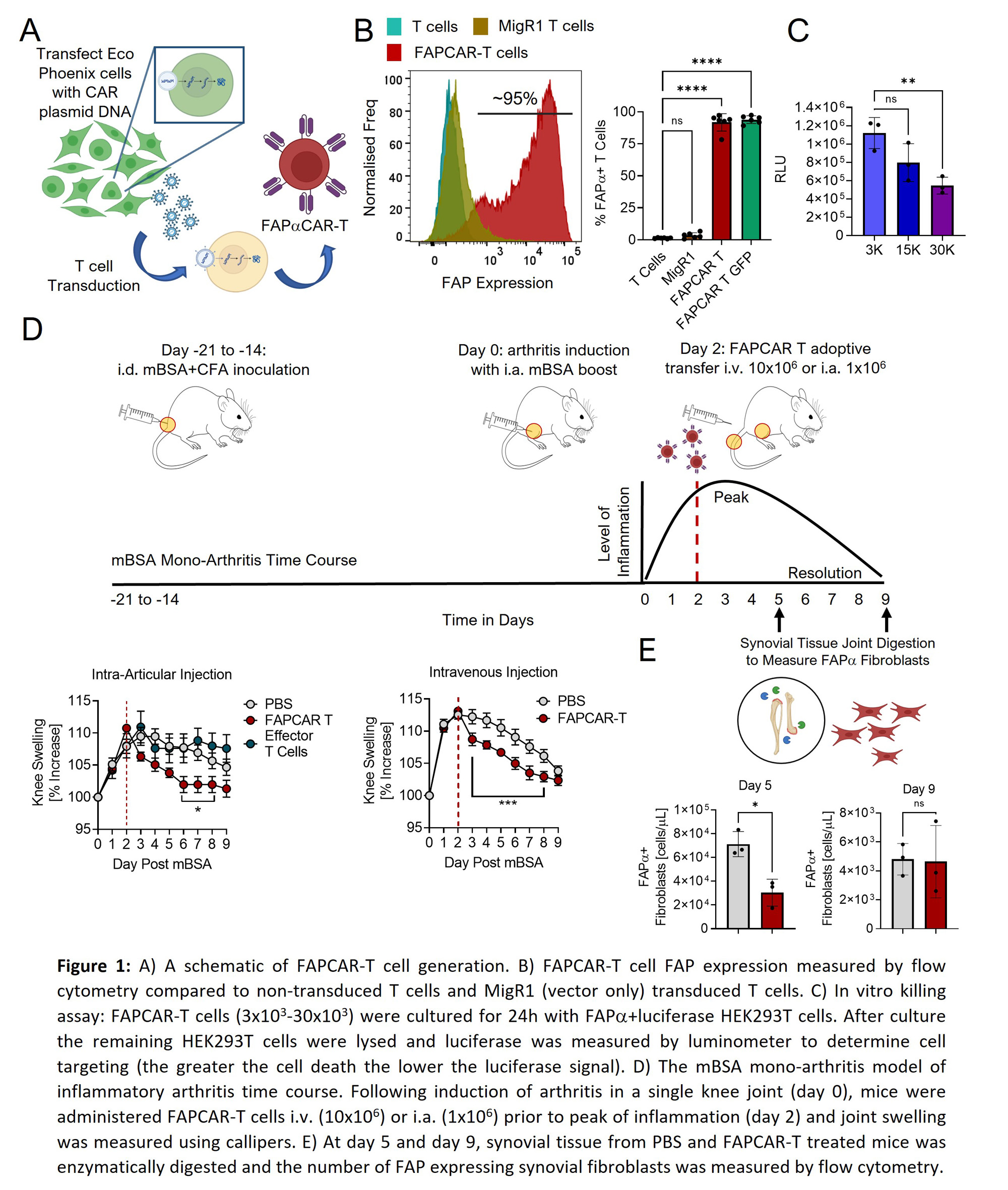
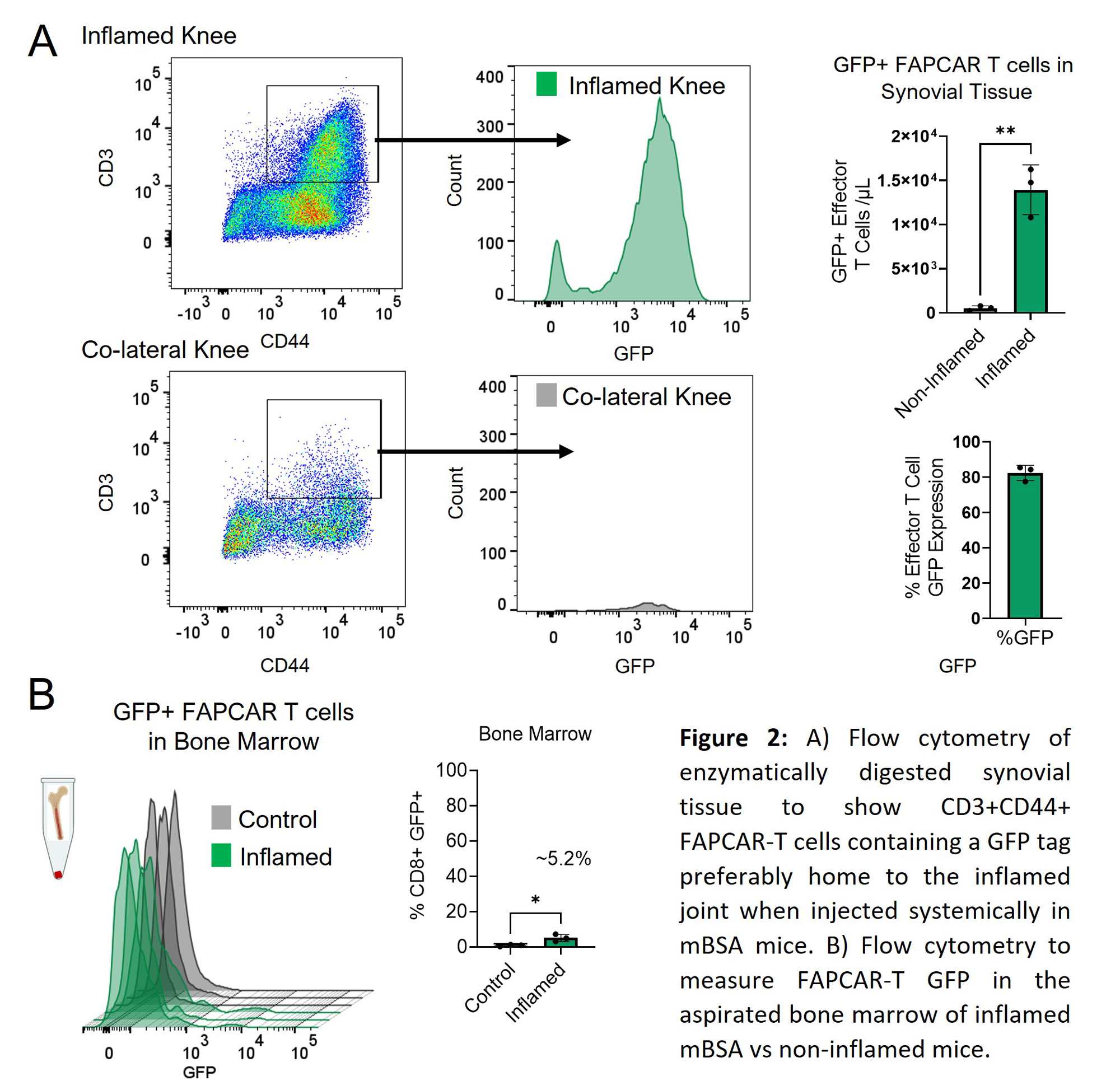
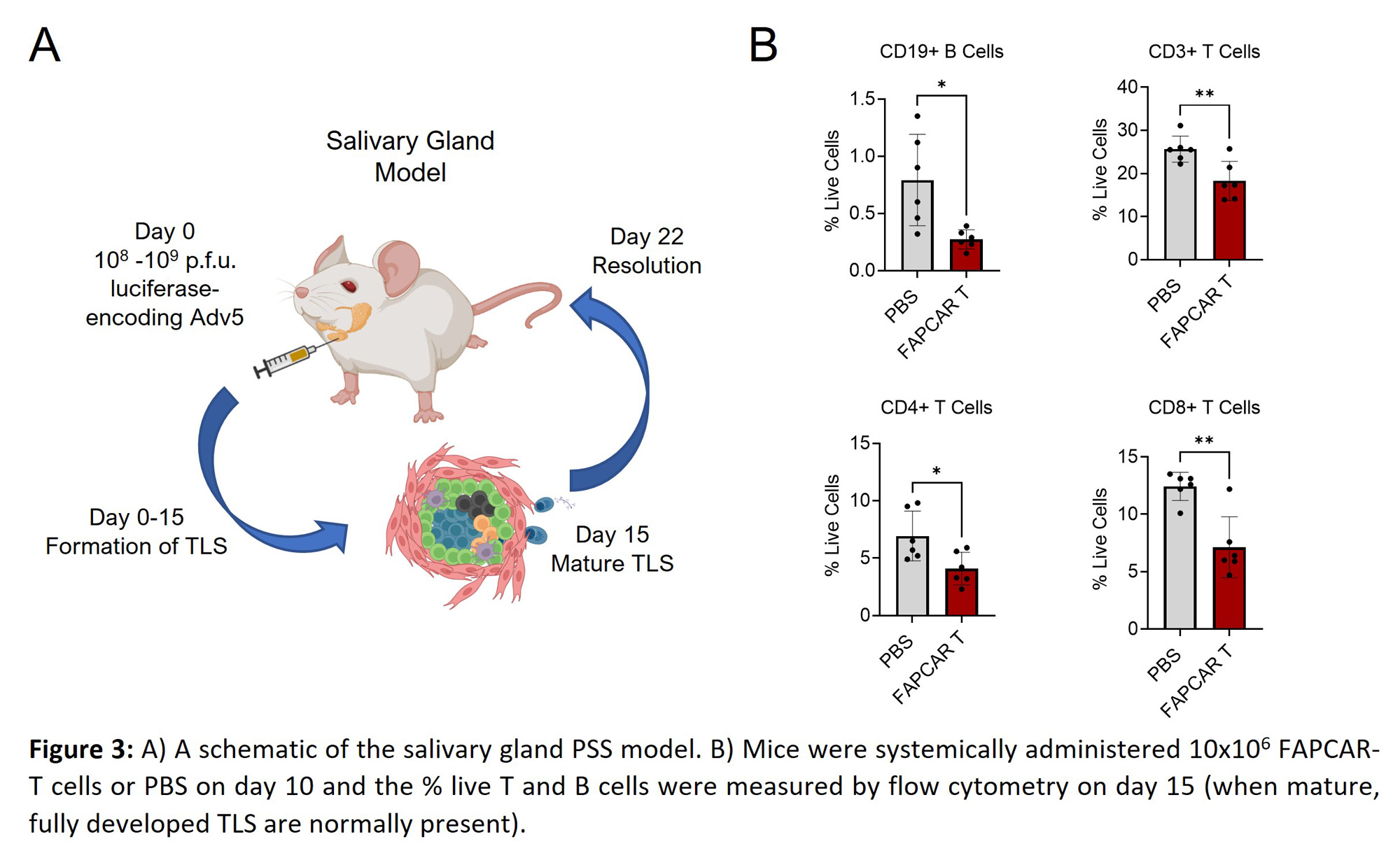
Methods: FAPCAR-T cells were generated and functionally tested as previously described (Aghajanian et al., Nature 2019) and were administered systemically and/or locally prior to peak of inflammation in murine models of inflammatory arthritis, or an inducible salivary gland inflammation model. Arthritis severity was determined by clinical scores and caliper measurements of swollen joints and changes in fibroblast activation states using 3′ single cell RNA profiling of CD45- sort-purified, CAR-treated synovial tissue. TLS characterization was determined by immunohistochemistry. FAPCAR-T-GFP, Fibroblast FAPa expression and leukocyte infiltration in enzymatically digested synovial or salivary gland tissue was measured by flow cytometry to determine CAR-T homing, FAPa targeting and inflammation, respectively.
Results: In arthritis, targeted deletion of FAPa fibroblasts was achieved through the adoptive transfer of FAPCAR-T cells that homed to the site of inflammation upon systemic administration and resulted in significant FAPa fibroblast cell depletion in the inflamed tissue. In both mono-articular and polyarticular arthritis models the intravenous or direct intra-articular administration of FAPCAR-Tcells prior to peak of inflammation suppressed disease severity by significantly attenuating joint inflammation. Single cell RNA profiling of CD45- sort-purified, FAPCAR-T treated synovial tissue identified a global defect in the stromal cell landscape. Systemic administration of FAPCAR-T cells in the salivary gland model significantly decreased the proportion of T and B cells and influenced TLS formation and maturity.
Conclusion: FAPα is an attractive, therapeutically targetable biomarker of pathogenic fibroblasts. This study demonstrates potential therapeutic efficacy of fibroblast-targeted immunotherapy as a novel treatment in inflammatory disease.
To cite this abstract in AMA style:
Kemble S, Mahony C, Smith C, Rurik J, Aghajanian H, Epstein J, Coles M, Croft A. Targeting Fibroblasts in Inflammatory Disease Using Engineered T Cells [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/targeting-fibroblasts-in-inflammatory-disease-using-engineered-t-cells/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-fibroblasts-in-inflammatory-disease-using-engineered-t-cells/