Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Impaired macrophage efferocytosis, a key hallmark of failed inflammation resolution, is reported in many immune-mediated inflammatory diseases, including in Rheumatoid Arthritis (RA). Hence the restauration of macrophage function and with that driving inflammation resolution, through modulating immune effector cells and inducing tissue repair is an attractive approach. The objective of the present study was to evaluate if macrophages can be targeted for inflammation resolution using an autologous secretome prepared from resolving macrophage in preclinical models and on RA patients’ blood samples.
Methods: Pre-clinical testing was performed in the collagen-induced arthritis (CIA) model. PBMCs from CIA mice with or without standard of care treatments, RA patients or healthy donors (HD) (NCT02839278) were isolated and differentiated into phagocytes (M-CSF for 7 days). After conditioning into resolving macrophages, respective secretomes were collected and filtered to exclude cellular debris. Mouse secretomes were injected and arthritis was monitored over 10 days. On human blood samples, cytokines and lipid mediators were quantified using CBA multiplex and MS analysis, respectively, and efferocytic capacities were measured by flow cytometry. Prepared RA patient secretomes were evaluated for their pro-resolving properties using a monocyte activation test, evaluating the reduced release of TNFa.
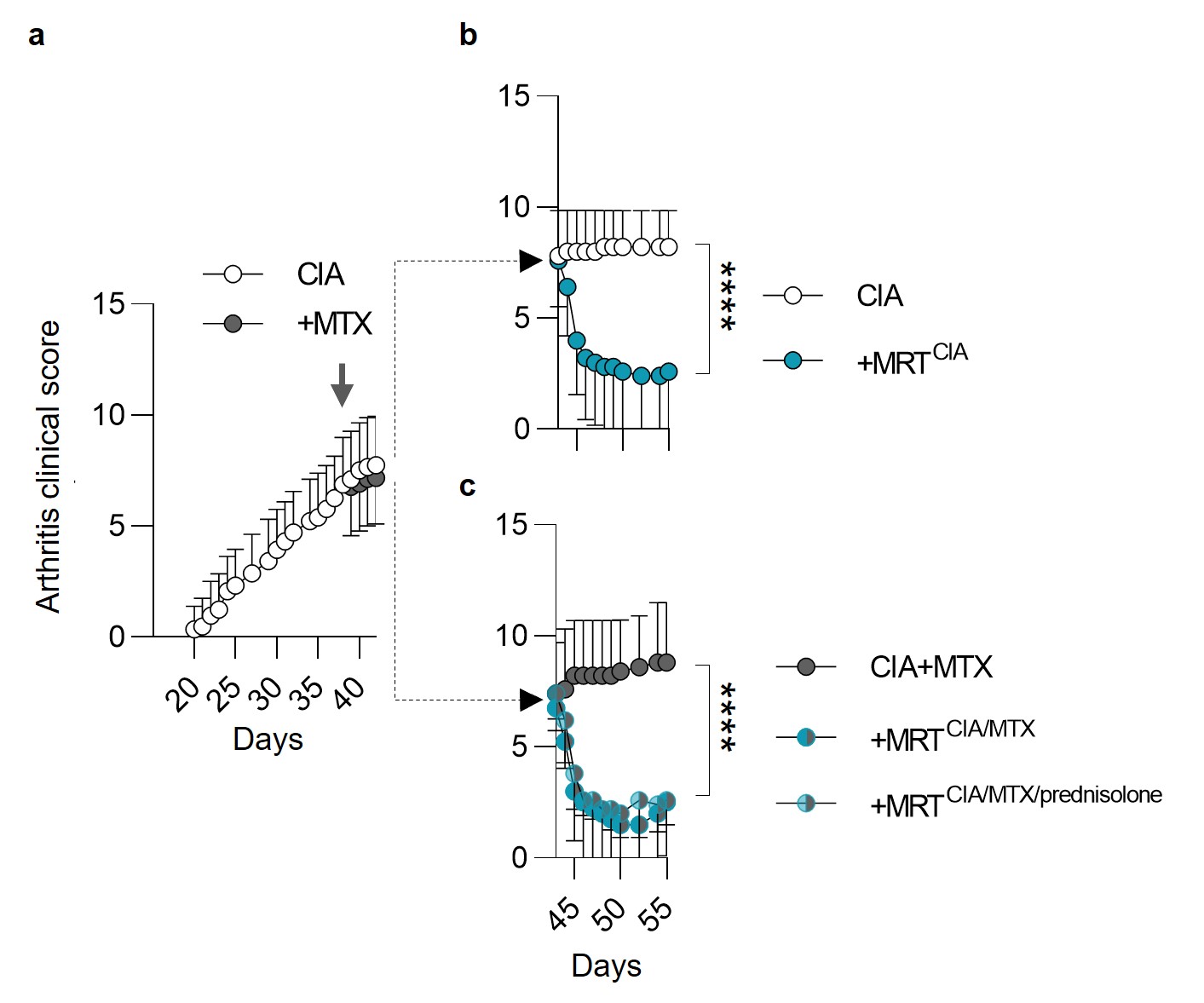
Results: To closely mirror a RA patient setting, we prepared autologous resolving macrophage secretomes from CIA diseased mice (MRTCIA), which after a single dose injection, demonstrated potent effects, in reversing disease activity (Fig 1a, b) which was significantly superior to the effect of methotrexate (MTX) (Fig 1c). Furthermore, the potent therapeutic activity was unaffected using secretomes obtained from animals receiving conventional treatments (methotrexate +/- prednisolone). These therapeutic effects were depending on the presence of myeloid cells and accompanied with the reprogramming of tissue macrophages for increased efferocytosis, inhibition of monocyte activation and an increase of regulatory T cells (not shown), confirming the reinitiation of resolution.
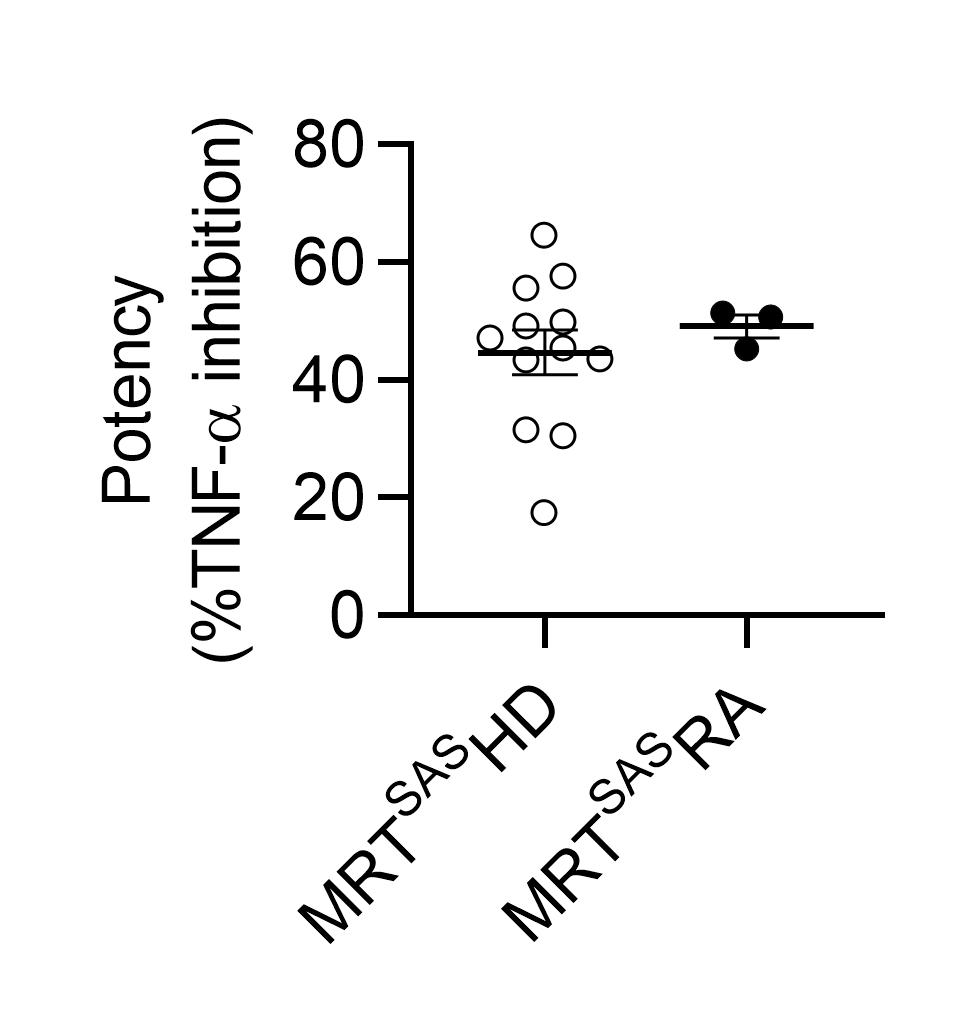
When analyzing RA patient samples (N = 35) versus HD (N = 31), we confirmed the inflammatory profile through the detection of increased levels of pro-inflammatory cytokines (TNF-a, IL-6, IL-8, IL-1β and IL-12) and omega-3 fatty acid-derived pro-inflammatory lipid mediators. Patients monocytes and dendritic cells presented higher levels of the co-stimulatory marker CD40. Interestingly, when outside of the inflammatory environment, monocyte-derived macrophages demonstrated similar efferocytic capacities compared to that of HD and more importantly, resulting resolving macrophage secretomes showed similar monocyte-inhibitory activities than that of HD (Fig 2).
Conclusion: Our translational and pre-clinical data show unprecedented disease modifying activities of autologous resolving macrophage secretomes. This approach will be tested as a next generation disease modifying modality/bDMARDs in RA patients starting in 2025.
To cite this abstract in AMA style:
Couturier M, Bonnefoy F, Gaiffe e, Vauchy C, Behlke S, TOUSSIROT E, Perruche S. Targeting Defective Macrophages to Restore Resolution of Inflammation in Rheumatoid Arthritis -Perspectives for an Autologous Secretome Therapy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/targeting-defective-macrophages-to-restore-resolution-of-inflammation-in-rheumatoid-arthritis-perspectives-for-an-autologous-secretome-therapy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-defective-macrophages-to-restore-resolution-of-inflammation-in-rheumatoid-arthritis-perspectives-for-an-autologous-secretome-therapy/