Session Information
Date: Monday, November 14, 2022
Title: Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Gout is the most common type of inflammatory arthritis, characterised by chronic deposition of uric acid crystals in the joints, affecting approx. 1-2% of the adult population in the Czech Republic. Currently, it is not possible to predict whether patients with hyperuricemia (HUA) will develop gout, to evaluate the risk of recurrent attacks, or joint damage. Impaired cardiovascular and kidney functions have recently been more precisely described in gout patients. Identification of novel biomarkers of gout pathogenesis is desirable for prevention and risk stratification of groups susceptible to acute attacks of gouty arthritis, development of joint damage, and important comorbidities.
Methods: In this study, a targeted lipidomic analysis consisting of high-performance liquid chromatography coupled with mass spectrometry was used to analyse plasma of clinically and biochemically characterized gout (n=194) and HUA (n=96) patients and a normouricemic control group (n=70), described previously [1, 2]. Statistical analysis included univariate approaches (t‑test, ROC analysis) and multivariate approaches (PCA, OLPS-DA). Data visualization was performed using the Cytoscape software to construct lipidome maps.
References:
1. Horváthová et al. J Clin Med. (2019) 8(11):1965.
2. Bohatá et al. Arthritis Res Ther . (2021) 10;23(1):186.
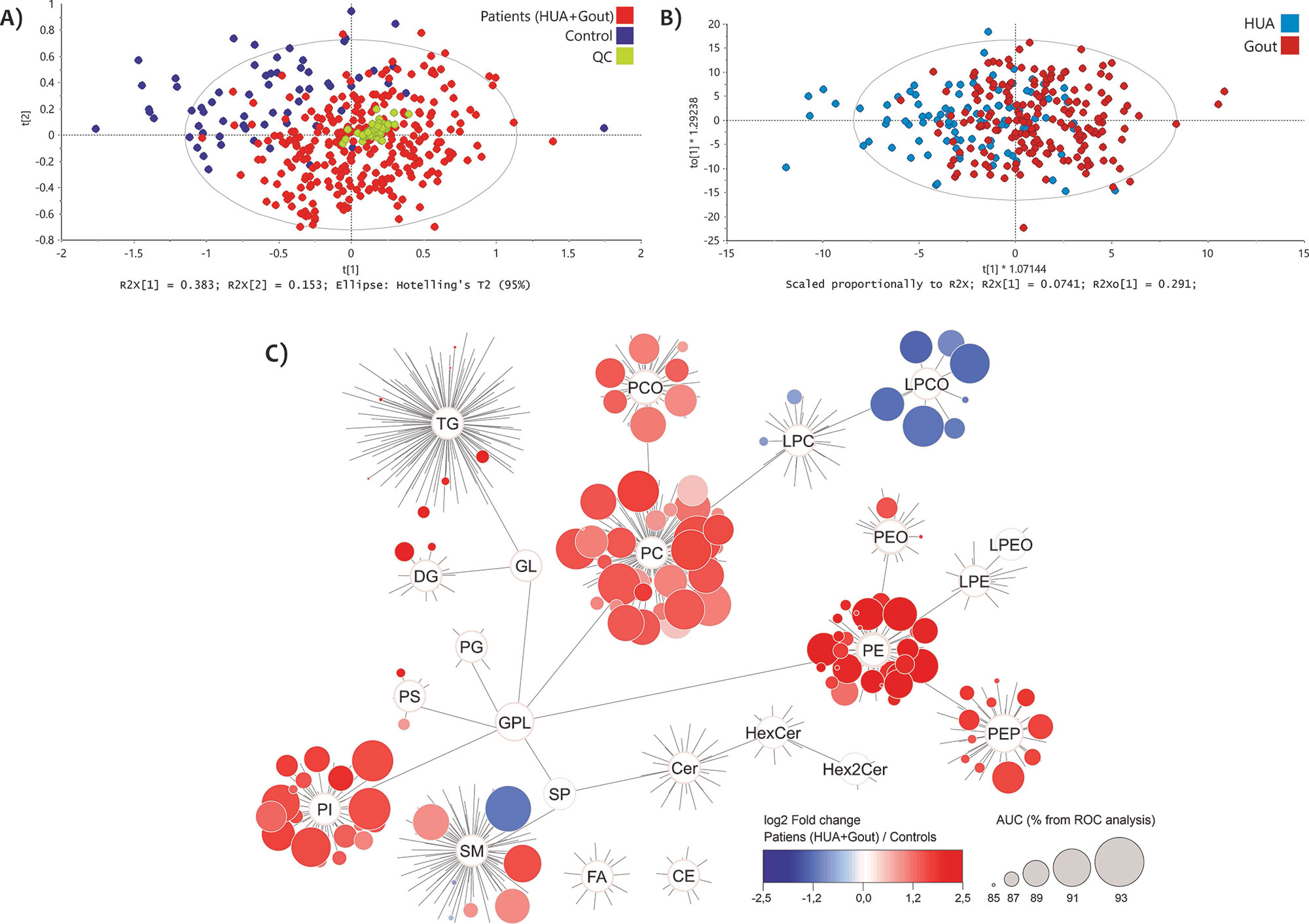
Results: More than 650 lipids belonging to 20 lipid (sub)classes were measured and semiquantified in plasma. Statistically significant discrimination of the study groups was observed by un/supervised statistical analyses. In patients, increased levels of phosphatidylinositols, phosphatidylethanolamines and phosphatidylcholines were observed. On the other hand, lysophosphatidylcholines (LPC) and their plasmalogens (LPC-O) were decreased in patients. Clinical performance of selected lipid markers exceeded 90% AUC (from ROC) analysis for more than 30 lipids
Conclusion: Our results point to the involvement of multiple glycerophospholipid classes, for instance LPC and LPC-O, in gout pathobiochemistry. To elaborate, a proinflammatory effect of LPC has been described in the pathogenesis of atherosclerosis. LPC‑O can serve as oxygen and radical scavengers via their vinyl ether bond which is reactive to oxygen and nitrogen species in the context of inflammation-induced oxidative stress. These results will help to further understand the pathobiochemical mechanism of gout and HUA, with the potential for early detection of gout-susceptible HUA patients.
Multivariate statistical models based on 659 variables (identified lipids). A) Principal component analysis of gout and hyperuricemic (HUA) patients (red) vs. controls (blue) and quality control samples (QC, green), B) OPLS-DA for gout patients (dark red) vs. HUA (dark blue). C) Map of the lipidome of 20 lipid (sub)classes as a comparison between all patients (HUA+Gout) against controls. Each circle symbolizes a unique lipid represented by the change in median (log2 fold change, blue – decreased, red – increased in patients) and AUC value in percentage by increasing size (from ROC analysis) for the group of patients versus controls. Lipids below 85% AUC are not visualized.
To cite this abstract in AMA style:
Stiburkova B, Pavelcová K, Bohatá J, Pavelka K, Hasíková L, Závada J, Kvasnička A, Dobešová D, Friedecký D. Targeted Plasma Lipidomics Distinguishes Patients with Gout and Hyperuricemia from Controls [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/targeted-plasma-lipidomics-distinguishes-patients-with-gout-and-hyperuricemia-from-controls/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeted-plasma-lipidomics-distinguishes-patients-with-gout-and-hyperuricemia-from-controls/