Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
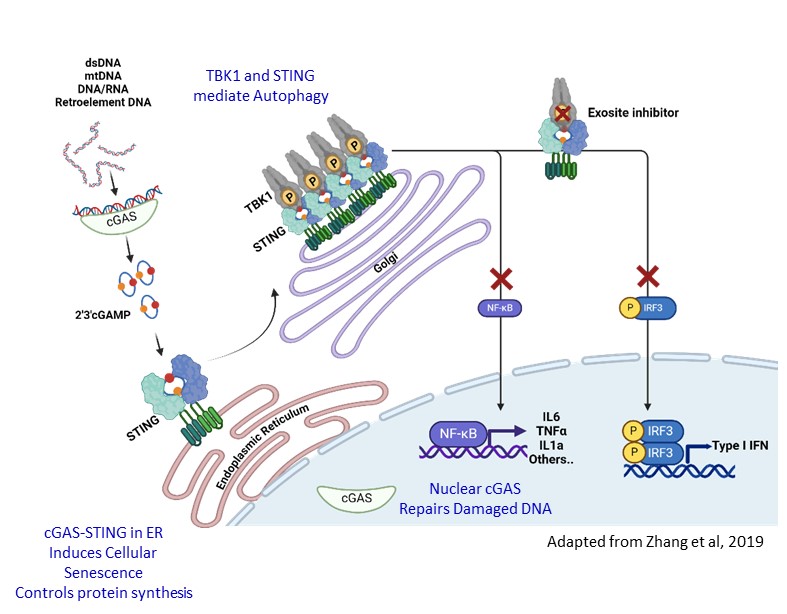
Background/Purpose: The cGAS-TBK1-STING pathway senses nucleic acids for innate immunity. Aberrant activation of the pathway is linked to autoimmune diseases including Systemic and Cutaneous Lupus (SLE and CLE), Systemic Scleroderma (SSc), and Sjögren’s Syndrome. To overcome the existing challenges to inhibit this pathway, we discovered novel “exosite” inhibitors of TBK1 (EXO-TBKi) that disrupt STING-mediated TBK1 activation. EXO-TBKi potently and selectively inhibit pathogenic effects of TBK1-STING (Type-I IFN, NFkB, and markers of fibrosis), while sparing desirable antiviral and autophagy functions to improve the therapeutic window of TBK1 inhibition. We evaluated the efficacy of EXO-TBKi for inhibition of inflammatory signals in cellular, murine models and patient samples.
Methods: Utilizing a proprietary platform, we identified a plausible druggable exosite pocket, with biological function to inhibit STING mediated TBK1 activation. Incorporation of exosite specific assays in the ligand-finding campaign led to discovery of TBK1 selective exosite series. Rational and structure-based drug design resulted in identification of novel TBK1 inhibitors with exquisite binding affinity to exosite pocket. EXO-TBK1i were characterized in immortalized and primary immune cellular models for inhibition of Type-I IFN, NFkB markers upon STING activation. To investigate efficacy in a disease setting, we evaluated activity in SLE patient derived whole blood and PBMC, and SSc patient derived fibroblasts. Additionally, we assessed impact of EXO-TBK1i in TREX1 knock out mouse model, a lupus relevant model of interferonopathy.
Results: EXO-TBK1i display exquisite exosite binding affinity (IC50 < 0.009 µM), that translates into nM potencies in cells (THP1, primary human monocytes and mouse macrophages) measuring IFN responses upon STING activation. No kinase activity detected when profiled in a kinome panel. Compounds display dose-dependent inhibition of p-TBK1, p-STING, p-IRF3 and p-P65 in THP1 cells. EXO-TBK1i demonstrate differential activity compared to TBK1 active site and STING inhibitor. EXO-TBK1i preserve autophagy, and spare RIG-I/TBK1 activity with 100-fold selectivity. Dose dependent suppression of pSTING and pTBK1 and inflammatory cytokines observed in a DMXAA mouse model. EXO-TBKi robustly inhibit Type-1 IFN and NFkB activation in SLE patient whole blood and PBMC. Similarly, EXO-TBK1i significantly suppressed pathway activation, and expression of disease associated markers in SSc patient derived and healthy fibroblasts. Further, when dosed orally, EXO TBK1i suppresses heart inflammation in TREX1 knock out mice, with dramatic reduction of IFNβ, CXCL-10, CXCL-9 and IFIT1.
Conclusion: We discovered novel TBK1 inhibitors that selectively and potently inhibit the TBK1-STING pathway via specific inhibition of interferon and NF-κB while sparing the non-disease relevant functions of the kinase, a first-in-class mechanism that offers highest therapeutic benefit. We demonstrate efficacy across preclinical models and are progressing towards developmental candidate nomination for clinical opportunity in auto-immune diseases such as lupus, SSc and Sjogren’s.
To cite this abstract in AMA style:
Martin M, Wilker E, Gikunju D, Narayanan U, Pandya U, Prakash V, Edwards A, Kawatkar S, Chen T, Chandran R, Sunder S, Biradar S, Distler J, Joseph A, Ioannidis S, Vangamudi B. Targeted Exosite Inhibition of STING Activation of TBK1 Selectively Blocks Type I Interferon and NFκB Responses for Treatment of Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/targeted-exosite-inhibition-of-sting-activation-of-tbk1-selectively-blocks-type-i-interferon-and-nf%ce%bab-responses-for-treatment-of-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeted-exosite-inhibition-of-sting-activation-of-tbk1-selectively-blocks-type-i-interferon-and-nf%ce%bab-responses-for-treatment-of-autoimmune-diseases/