Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Our study aimed to use machine-learning approaches to characterize the immune cell profiles of patients who were inadequate responders to Etanercept-Biosimilar Yisaipu (Yisaipu-IRs) and develop an Yisaipu-IR risk model based on immune cell signatures in rheumatoid arthritis (RA).
Methods: The study included RA patients from the Department of Rheumatology and Immunology, Peking University People’s Hospital between July 1, 2022 and May 3, 2023. All the patients fulfilled the 2010 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) classification criteria for RA. All the patients received Yisaipu 25mg twice a week for 6 months. Patients who didn’t achieve ACR20 improvement at 3rd month after enrollment were defined as Yisaipu-IRs and others were defined as Yisaipu adequate responders (Yisaipu-ARs). Immunophenotyping profiles (24 immune cell subsets) of peripheral blood mononuclear cell from Yisaipu-IRs and Yisaipu-ARs were determined by flow cytometry. Spearman correlation analysis was used to test the association between immune phenotypes. Sparse partial least squares-discriminant analysis (sPLS-DA) was used to assess classification and parameter selection. Univariate and multivariate logistic regression analyses were performed to identify immune cell signatures associated with Yisaipu-IRs to build a risk model. Study design and analysis plan flow diagram was shown in figure1.
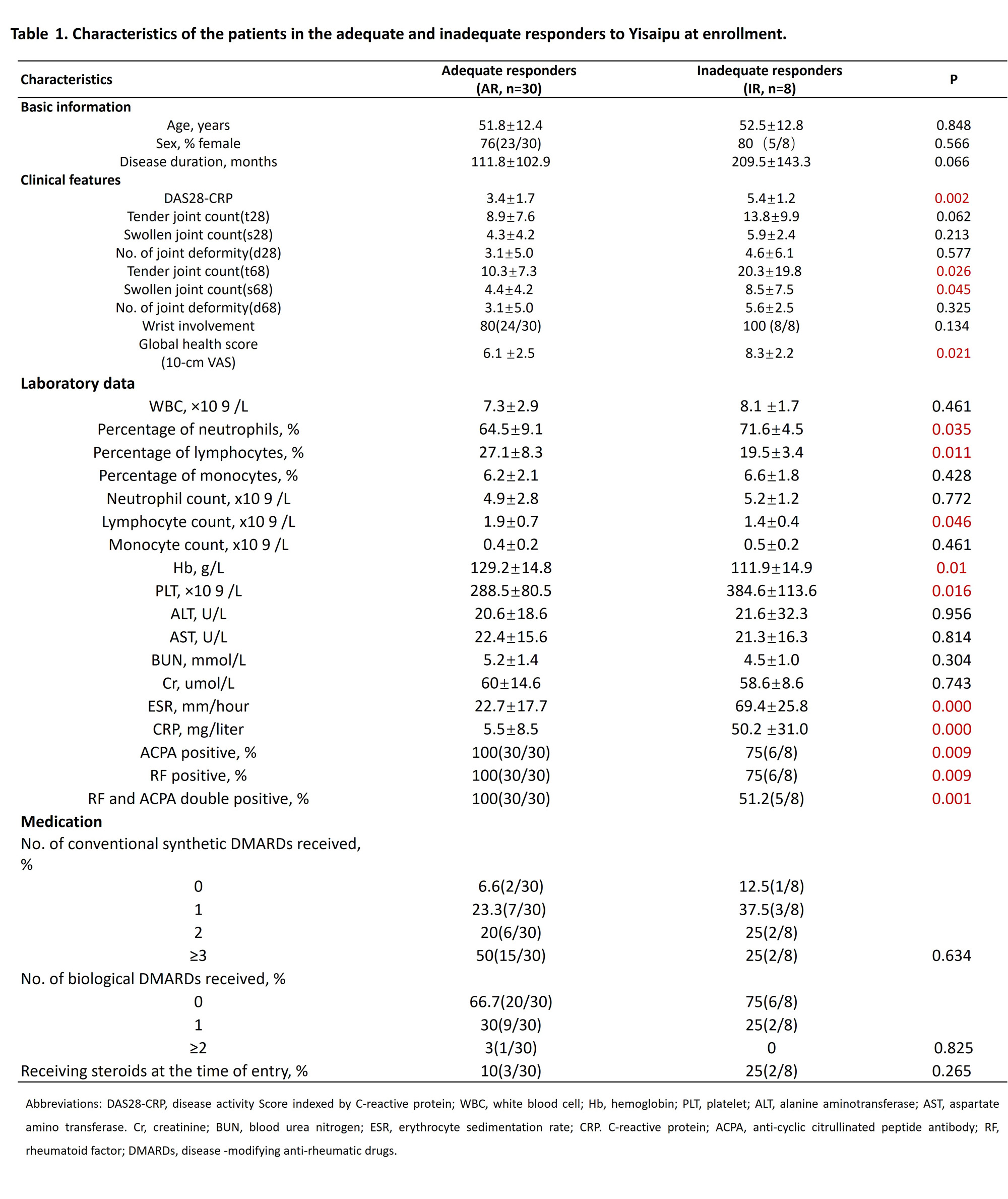
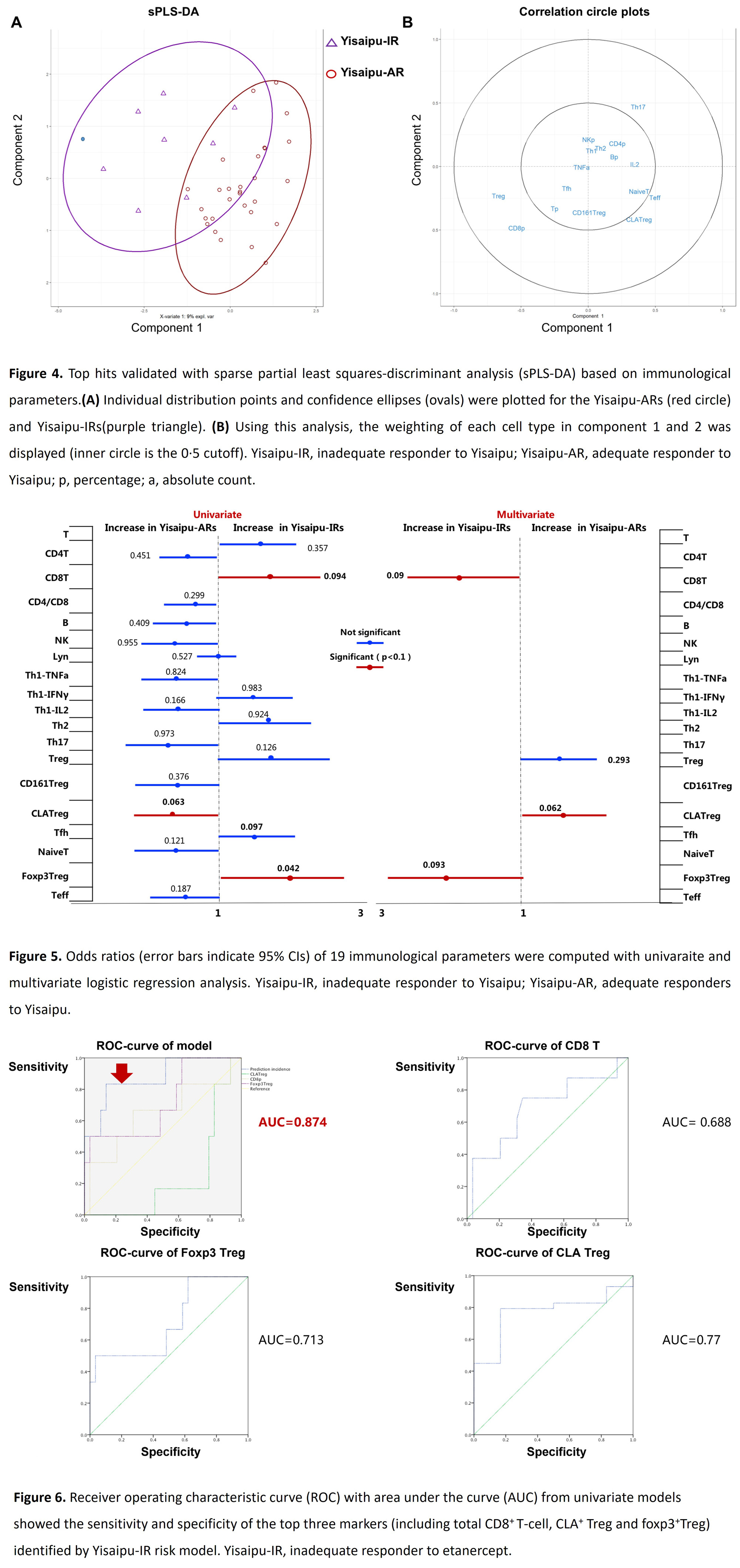
Results: Peripheral blood was collected from 30 Yisaipu-ARs and 8 Yisaipu-IRs. The demographic data and clinical features of the patients were described in table 1. The mean age was 51.8±12.4 years for Yisaipu-ARs and 52.5±12.8 years for Yisaipu-IRs. In-depth immune cell phenotyping showed that Yisaipu-IRs had a disrupted immune cell profile, compared with Yisaipu-ARs, including alteration in total CD8+ T-cell, regulatory T-cell (Treg), effector T-cell (Teff) and total B-cell (figure 2). Correlation analysis displayed significant disruption in the relationship among total CD8+ T-cell, Treg and B-cell with each other in Yisaipu-IRs compared to Yisaipu-ARs (figure 3). The top-ranked immunological features discriminated by sPLS-DA included total CD8+ T-cell, Treg and CLA+ Treg and Teff (figure 4). Logistic regression analysis was applied to confirm that total CD8+ T-cell, CLA+ Treg and foxp3+Treg were significantly associated with ETN-IRs, substantiating the global immunological difference between Yisaipu-IRs and Yisaipu-ARs (figure 5). A multi-parametric prediction model for Yisaipu-IRs was established based on weighted immunological signatures including total CD8+ T-cell, CLA+ Treg and foxp3+ Treg. Receiver operating characteristic curve (ROC) analysis of the model showed an area under the curve (AUC) of 0.874 (accuracy 87.4%) (figure 6), indicating good performance in discriminating Yisaipu-IRs from Yisaipu-ARs.
Conclusion: T cell signatures might promisingly predict the clinical response to Yisaipu in RA patients and facilitate better stratification of patients for optimal treatment choices in the future.
To cite this abstract in AMA style:
Zhu H, Tang S, Cheng G, Li Y, Li Y, Sun F, Sun X, Cheng J, Li R, Li Z. T Cell Subset Signatures Predicted Clinical Response to Etanercept-biosimilar Yisaipu in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/t-cell-subset-signatures-predicted-clinical-response-to-etanercept-biosimilar-yisaipu-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/t-cell-subset-signatures-predicted-clinical-response-to-etanercept-biosimilar-yisaipu-in-patients-with-rheumatoid-arthritis/