Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Current ACR guidelines recommend the use of IACS for short-term acute pain relief in patients (pts) with knee OA.1 Bilateral knee OA can occur concurrently or subsequently develop in 80-90% of pts with unilateral disease.2,3 These pts may benefit from simultaneous IACS treatment of both knees. Triamcinolone acetonide extended-release (TA-ER; formerly FX006) is an extended-release, microsphere-based formulation of triamcinolone acetonide (TA) recently approved by the FDA for knee OA pain.4 In a phase 2 study, a single TA-ER injection demonstrated reduced systemic TA exposure relative to standard TA crystalline suspension (TAcs).5 Here, we assessed safety and systemic TA exposure following IA injection of TA-ER or TAcs into both knees in pts with bilateral knee OA.
Methods: In this phase 2, randomized, open-label study (NCT03378076), pts (≥40 years, BMI ≤40 kg/m2) meeting ACR clinical/radiographic criteria for bilateral knee OA received 2 IA injections (one in each knee) of TA-ER 32 mg (total 64 mg) or TAcs 40 mg (total 80 mg) and followed for 6 weeks. Safety was evaluated based on adverse events (AEs), physical exams, knee assessments, vital signs, and laboratory evaluations. Blood samples for plasma pharmacokinetics (PK) were collected at baseline (within 1 hour prior to injection), at Hours 1-6, 8, 10, and 12 postinjection, and on Days 2, 8, 15, 29, and 43. Plasma TA concentrations were assayed with a validated LC-MS/MS method.
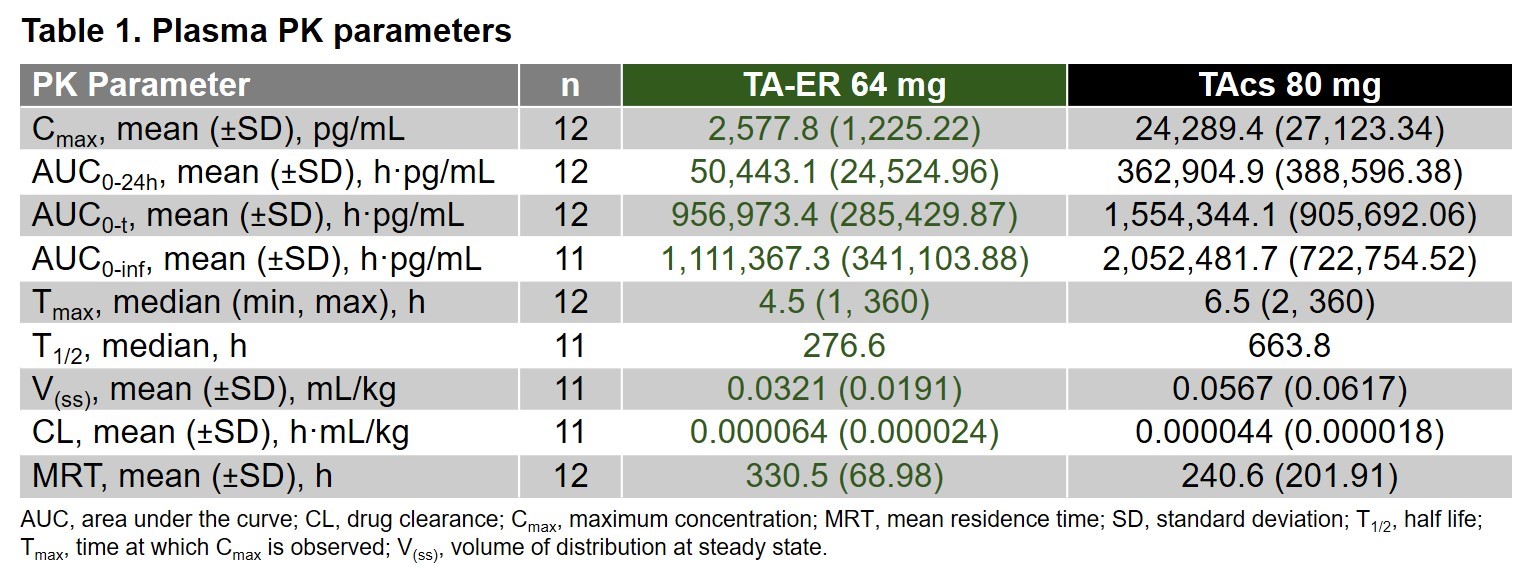
Results: Twenty-four pts (TA-ER, n=12; TAcs, n=12) were randomized and included in safety and PK analyses. Baseline characteristics were well balanced. Eight of 12 and 5 of 12 pts had ≥1 treatment-emergent AE in the TA-ER and TAcs treatment group, respectively. AE profiles were similar, and both treatments were well tolerated. TA-ER plasma concentrations peaked at median 4.5 hours with a mean Cmax (±SD) of 2577.8 (1225.22) pg/mL, whereas TAcs peaked at median 6.5 hours with a mean Cmax (±SD) of 24289.4 (27123.34) pg/mL (Figure 1 and Table 1).
Conclusion: In pts with bilateral knee OA, IA injection of TA-ER into both knees was generally safe and well tolerated. Peak plasma TA concentrations were substantially lower in patients treated with TA-ER, demonstrating reduced systemic exposure relative to TAcs consistent with the PK profile of a single TA-ER injection.
References:
1. Hochberg M et al. Arthritis Care Res (Hoboken). 2012;64(4):465-74
2. Metcalfe A et al. BMC Musculoskelet Disord. 2012;13:153
3. Jones R et al. J Rheumatol. 2013;40(3):309-15
4. Conaghan P et al. J Bone Joint Surg Am. 2018;100(8):666-77
5. Kraus V et al. Osteoarthritis Cartilage. 2018;26(1):34-42
To cite this abstract in AMA style:
Kivitz AJ, Kwong L, Shlotzhauer T, Lufkin J, Curto T, Kelley S. Systemic Exposure of Triamcinolone Acetonide Following Bilateral Injection of Extended-Release Triamcinolone Acetonide and Standard Triamcinolone in Patients with Bilateral Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/systemic-exposure-of-triamcinolone-acetonide-following-bilateral-injection-of-extended-release-triamcinolone-acetonide-and-standard-triamcinolone-in-patients-with-bilateral-knee-osteoarthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systemic-exposure-of-triamcinolone-acetonide-following-bilateral-injection-of-extended-release-triamcinolone-acetonide-and-standard-triamcinolone-in-patients-with-bilateral-knee-osteoarthritis/