Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) remains as the major driver of mortality in systemic sclerosis (SSc). The literature indicates that at the time of diagnosis of SSc-ILD or inclusion in clinical intervention studies the mean forced vital capacity (FVC) ranges from 66.5% to 72.6%. The VEDOSS project has demonstrated that more than 50% of patients with Raynaud’s and at least one “red flag” sign progress to fulfil ACR/EULAR 2013 criteria (A/E 2013) within 5 years. This data supports the notion of subclinical lung involvement very early within the natural history of SSc and a relative window for preventive intervention. Here we aimed to analyse the trajectory of FVC over time for the first time in the VEDOSS population in 2 independent cohorts to determine the presence and prevalence of subclinical lung volume loss in this population.
Methods: VEDOSS patients within our observational study STRIKE were screened for having an available FVC at baseline and at least one follow-up. Patients fulfilling the A/E 2013 at baseline were excluded. Lung function progress was assessed adapting the suggested progressive-fibrosis ILD (PF-ILD) definition, as per George et al. VEDOSS patients were considered to have a subclinical lung volume loss if they showed either a relative FVC decline of 10% or a 5% decline in FVC and a 15% decline in DLCO during follow-up. Progression to fulfil A/E 2013 was also considered in the analysis. These steps were then repeated in the separate EUSTAR VEDOSS database, with no patient overlap, analysed according to the EUSTAR CP140.
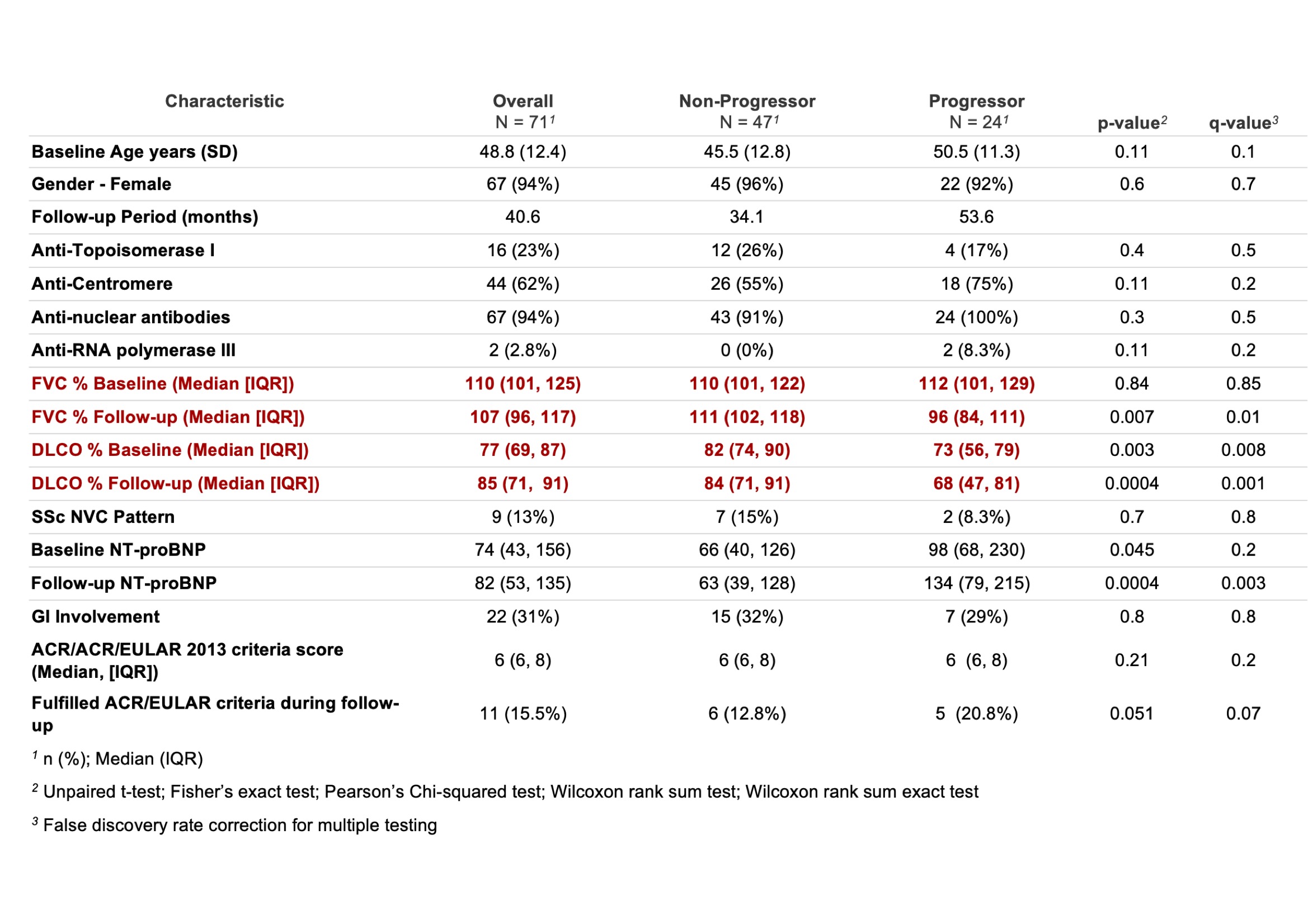
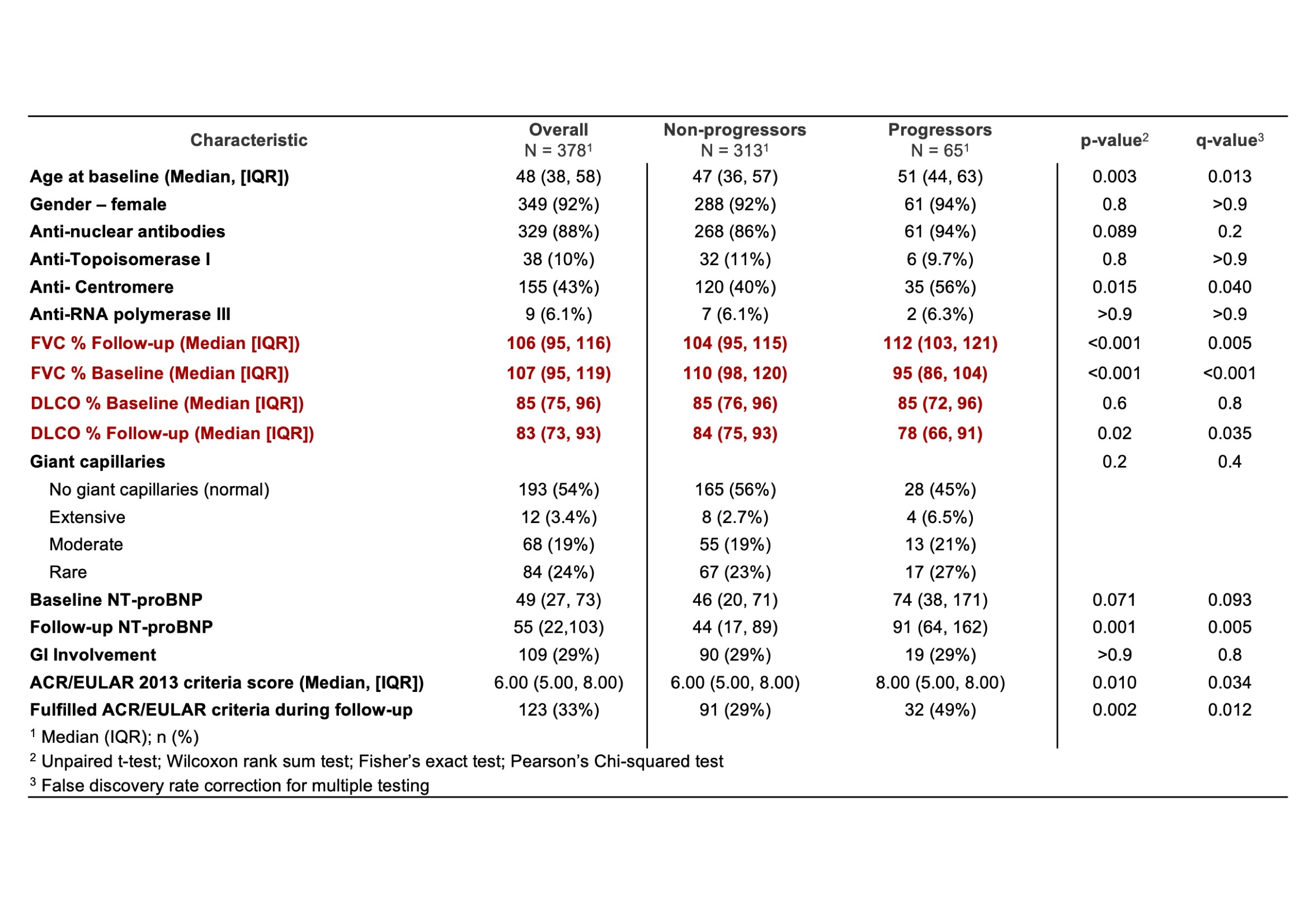
Results: 71 patients met the inclusion criteria in the STRIKE cohort. Baseline median FVC was 110% (101, 125) and median FVC at last available follow up was 107% (96, 117). Baseline DLCO was 77% (69, 87) and median DLCO at last available follow up was 85% (71, 91). 24 patients (33.9%) defined as sub-clinical progressors (sP), met the adapted PF-ILD criteria with a median baseline FVC of 112% (101, 129) and a median follow-up FVC of 96% (84, 111). Their median baseline DLCO was 73% (56, 79) and median follow-up DLCO 68% (47, 81) (Table 1). 5 (21%) sP also met the A/E 2013 vs 6 (16%) of the non-progressors (nP) (P = 0.05). In the EUSTAR VEDOSS database, 378 patients met the inclusion criteria. Baseline median FVC was 106% (95, 116) and median FVC at last available follow up was 107% (95, 119). Baseline median DLCO was 85% (75, 96) and median DLCO at last available follow up was 83% (73, 93). 65 patients (17.3%) defined as sP met the adapted PF-ILD criteria with a median baseline FVC of 112% (103, 121) and a median follow-up FVC of 95% (86, 104). Their median baseline DLCO was 85% (72, 96) and a median follow-up DLCO of 78% (66, 91) (Table 2). 32 (49%) sP also met the A/E 2013 vs 91/313 of the nP (29%) (P = 0.02).
Conclusion: Data from the 2 independent cohorts demonstrated subclinical lung volume loss in the VEDOSS population ranging from 17.3 to 33.9%. Less than half of the sP met A/E 2013 during follow up in either cohort, suggesting an unmet need in the very early detection of lung involvement in SSc. Further prospective research is warranted to identify the relation between subclinical volume loss and future development of ILD to inform preventive approaches in the very early population.
To cite this abstract in AMA style:
Kakkar V, Di Donato S, Bellando-Randone S, Bixio R, Matucci-Cerinic M, Allanore Y, Del Galdo F. Subclinical Loss of Lung Volumes in Very Early SSc: Evidence from Two Independent Cohorts in EUSTAR [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/subclinical-loss-of-lung-volumes-in-very-early-ssc-evidence-from-two-independent-cohorts-in-eustar/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subclinical-loss-of-lung-volumes-in-very-early-ssc-evidence-from-two-independent-cohorts-in-eustar/