Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
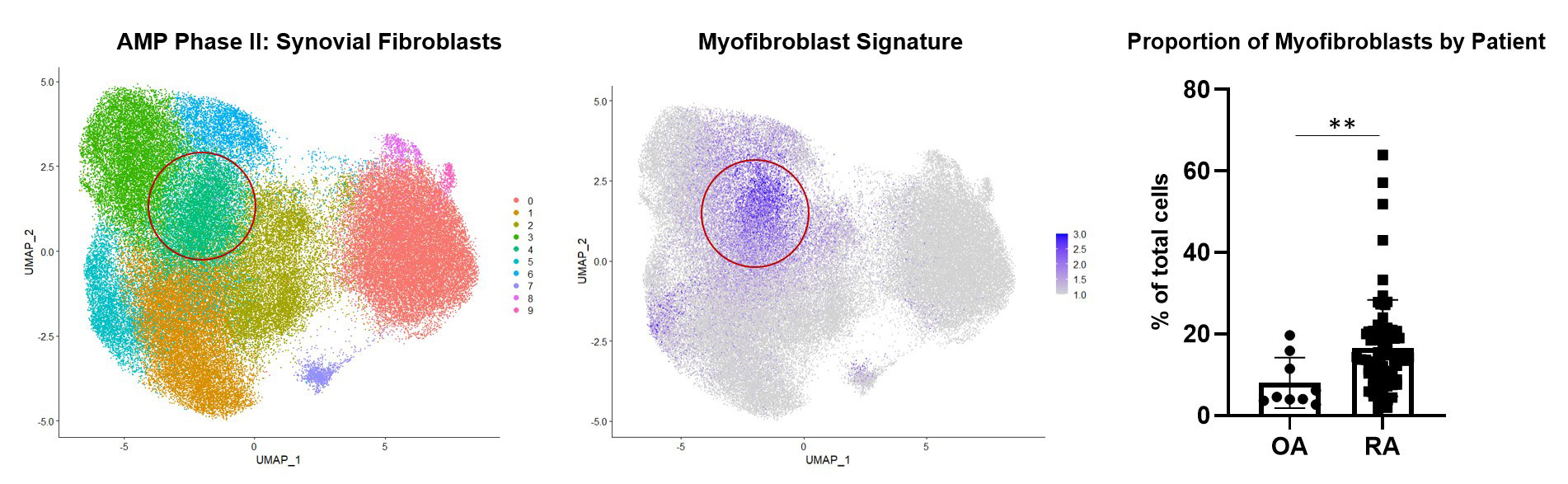
Background/Purpose: Many rheumatoid arthritis (RA) patients do not achieve sustained remission despite multiple lines of therapy. Recent studies have linked the expansion of synovial fibroblasts with treatment resistance in RA1. Using single-cell RNA-sequencing data generated by the Accelerating Medicines Partnership (AMP) Consortium, we identified the expansion of fibrotic myofibroblasts in RA distinguished by high expression of extracellular matrix proteins2. Here, we leveraged spatial transcriptomics and in vitro studies to reveal the vascular localization of myofibroblasts and identify the molecular drivers of this phenotype.
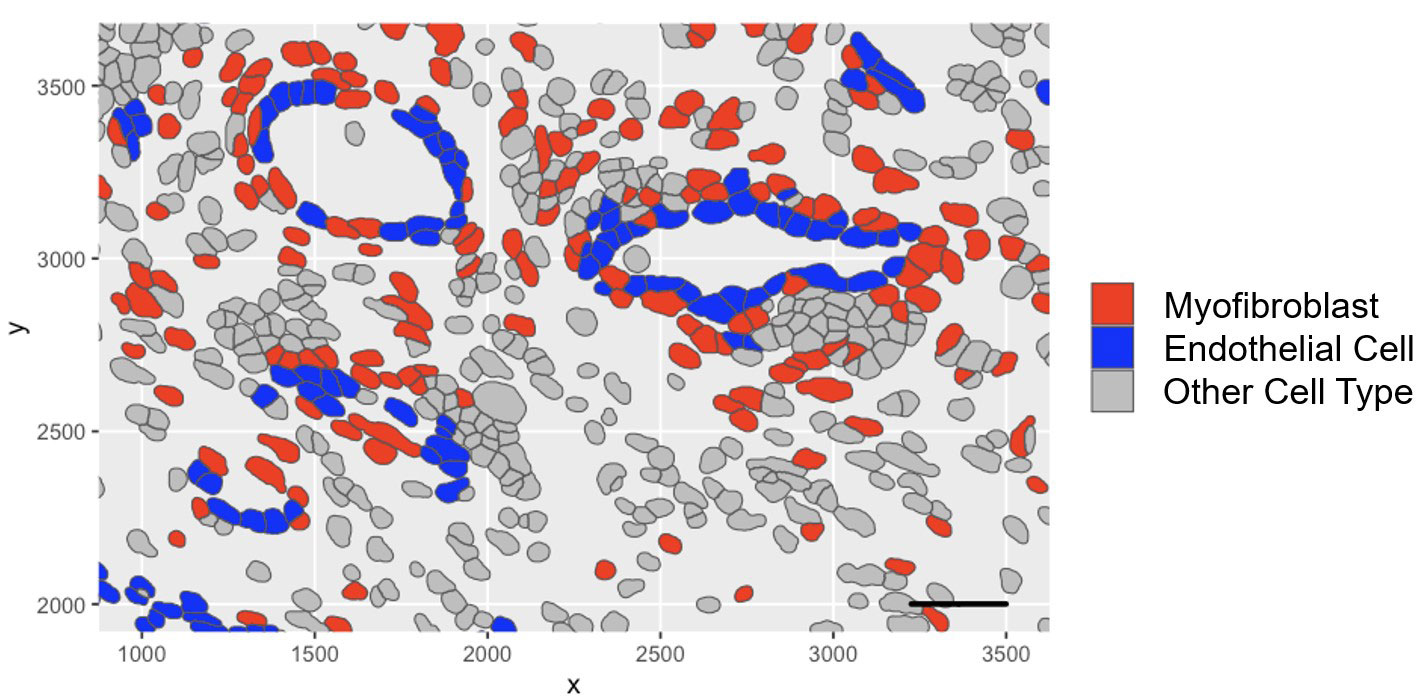
Methods: Spatial transcriptomic profiling: Synovial FFPE tissue sections (1 RA and 1 OA) were subject to cyclic in situ hybridization of 960 fluorescent mRNA probes and imaged using the CosMx™ Spatial Molecular Image (Nanostring). Cell segmentation was performed which was used to assign transcripts to cells. Subsequent clustering and visualization were performed.
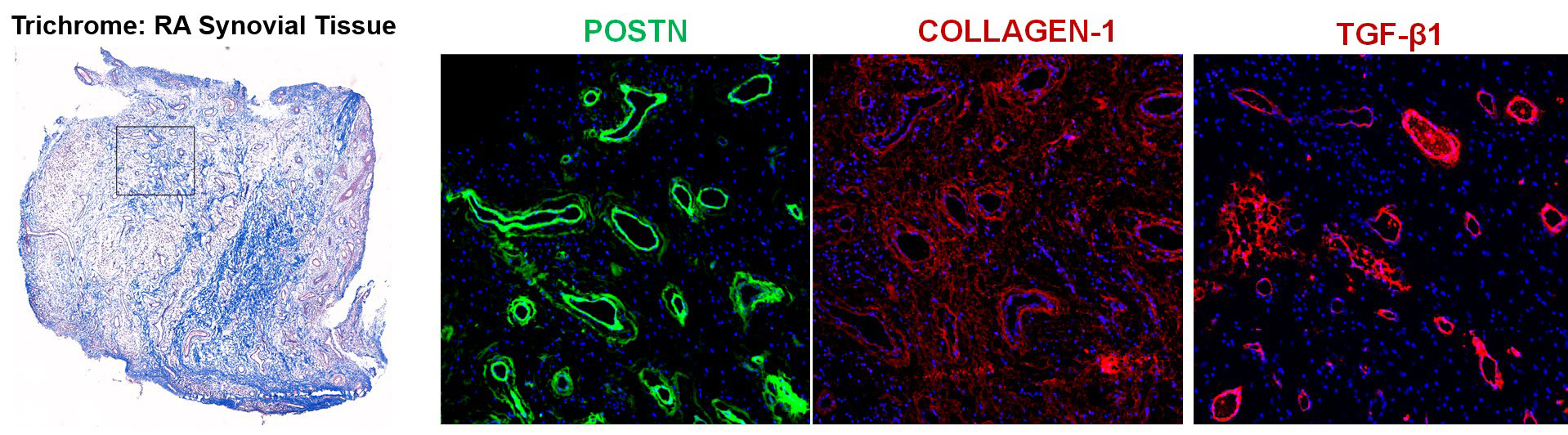
Immunofluorescent staining: RA and OA synovial tissue sections were stained with antibodies against CD31, NOTCH3, POSTN, COL1A1, p-SMAD2/3 and TGF-β followed by secondary antibody staining and image acquisition.
Cell culture: Synovial fibroblasts were cultured and stimulated with Notch ligand or co-cultured with human umbilical vein endothelial cells (HUVECs). qPCR, ELISA, western blot, and flow cytometry of stimulated cells were performed.
Results: Synovial scRNAseq data generated from 79 donors revealed the significant expansion of POSTN+ fibrotic myofibroblasts, defined by high expression extracellular matrix proteins COL1A1 and COMP, in RA. To identify the localization of myofibroblasts within RA synovia we applied a myofibroblast gene signature to spatial transcriptomic data which showed localization of myofibroblasts to the perivascular niche. Immunofluorescent staining of myofibroblast markers POSTN and COL1A1 confirmed the perivascular deposition of these proteins in RA synovia. Further scRNAseq analysis revealed a myofibroblast differentiation trajectory encompassing Notch and TGF-β signaling. Stimulation of synovial fibroblasts with Notch ligand delta-like ligand 4 and co-culture with HUVECs strongly induced the expression of POSTN. Unexpectedly, we found that Notch signaling promoted strong expression of TGF-β in vitro. Immunofluorescent staining of synovial tissue confirmed perivascular localization of NOTCH3, TGF-β and pSMAD2/3, suggesting a molecular crosstalk between Notch and TGF-β during differentiation of myofibroblasts in RA synovia.
Conclusion: Our study leveraged cutting-edge spatial transcriptomics to identify endothelial cells as mediators of synovial tissue fibrosis via cellular crosstalk involving Notch and TGF-β signaling. Further investigation into the molecular basis of myofibroblast differentiation in RA synovia will enable the identification of novel therapies targeting myofibroblasts in treatment resistant RA.
1. Rivellese, F et al. Nat Med, 19 May. 2022, doi:10.1038/s41591-022-01789-0
2. Zhang, F. et al. bioRxiv 2022.02.25.481990 (2022) doi:10.1101/2022.02.25.481990.
To cite this abstract in AMA style:
Bhamidipati K, Madhu R, Presti S, Kim Y, Cui Y, Zhang F, Jonsson A, Nathan A, Millard N, Rao D, Donlin L, Anolik J, Raychaudhuri S, Brenner M, Korsunsky I, Wei K. Spatial Transcriptomic Analysis Reveals Vascular Zonation of Myofibroblasts in Rheumatoid Arthritis Synovium [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/spatial-transcriptomic-analysis-reveals-vascular-zonation-of-myofibroblasts-in-rheumatoid-arthritis-synovium/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spatial-transcriptomic-analysis-reveals-vascular-zonation-of-myofibroblasts-in-rheumatoid-arthritis-synovium/