Session Information
Date: Saturday, November 16, 2024
Title: SLE – Animal Models Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Skin fibrosis is characterized by collagen deposition, fibro/myofibroblast accumulation, and extracellular matrix remodeling. Some subtypes of cutaneous lupus erythematosus (CLE), particularly discoid lupus erythematosus (DLE), feature prominent skin fibrosis with inadequate treatment options, often leading to disfigurement. However, mechanisms of scar formation in CLE and the role of the female sex-biased transcription coregulator VGLL3 in fibrosis are not understood. We have shown that epidermal-directed overexpression of murine Vgll3 causes fibrotic-appearing skin lesions suggestive of CLE. Thus, we aimed to explore Vgll3’s role in cutaneous fibrosis.
Methods: 3-month-old male and female transgenic (TG) mice overexpressing Vgll3 in the epidermis under the KRT5 promoter were compared to wildtype (WT) C57Bl/6 mice. Fibrotic biomarkers of human DLE and scleroderma were examined by immunostaining. In addition, single-cell-RNA-sequencing (scRNA-seq) from transgenic Vgll3 (Vgll3-TG) lesional and nonlesional and WT skin was performed to identify the transcriptomes of the potential cellular fibrotic players. Several subclusters of fibroblasts (FB), myofibroblasts, pericytes and T cells were identified, and expression of fibrotic compared. Cellchat (v2.1.2) was used for ligand-receptor analysis.
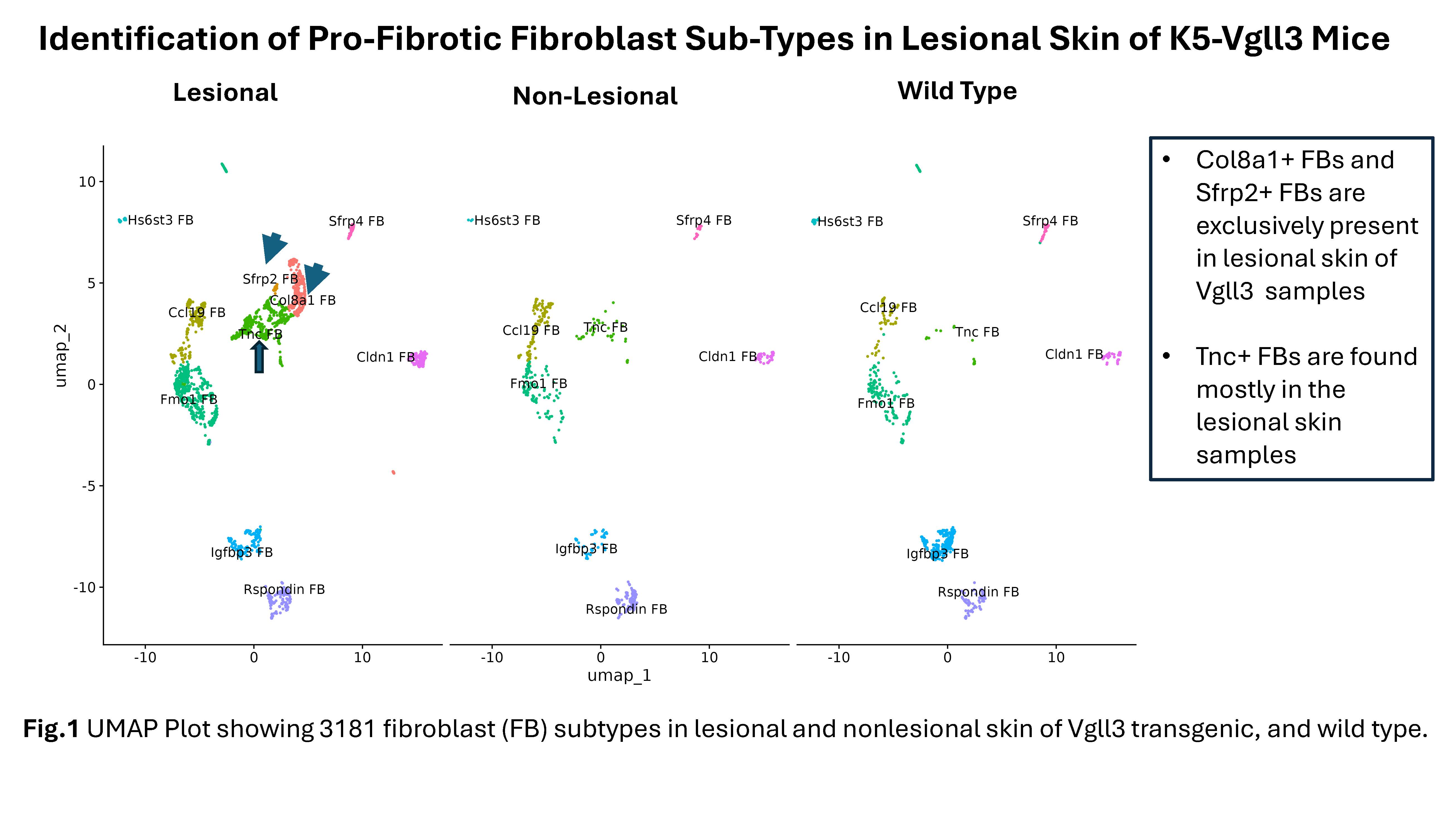
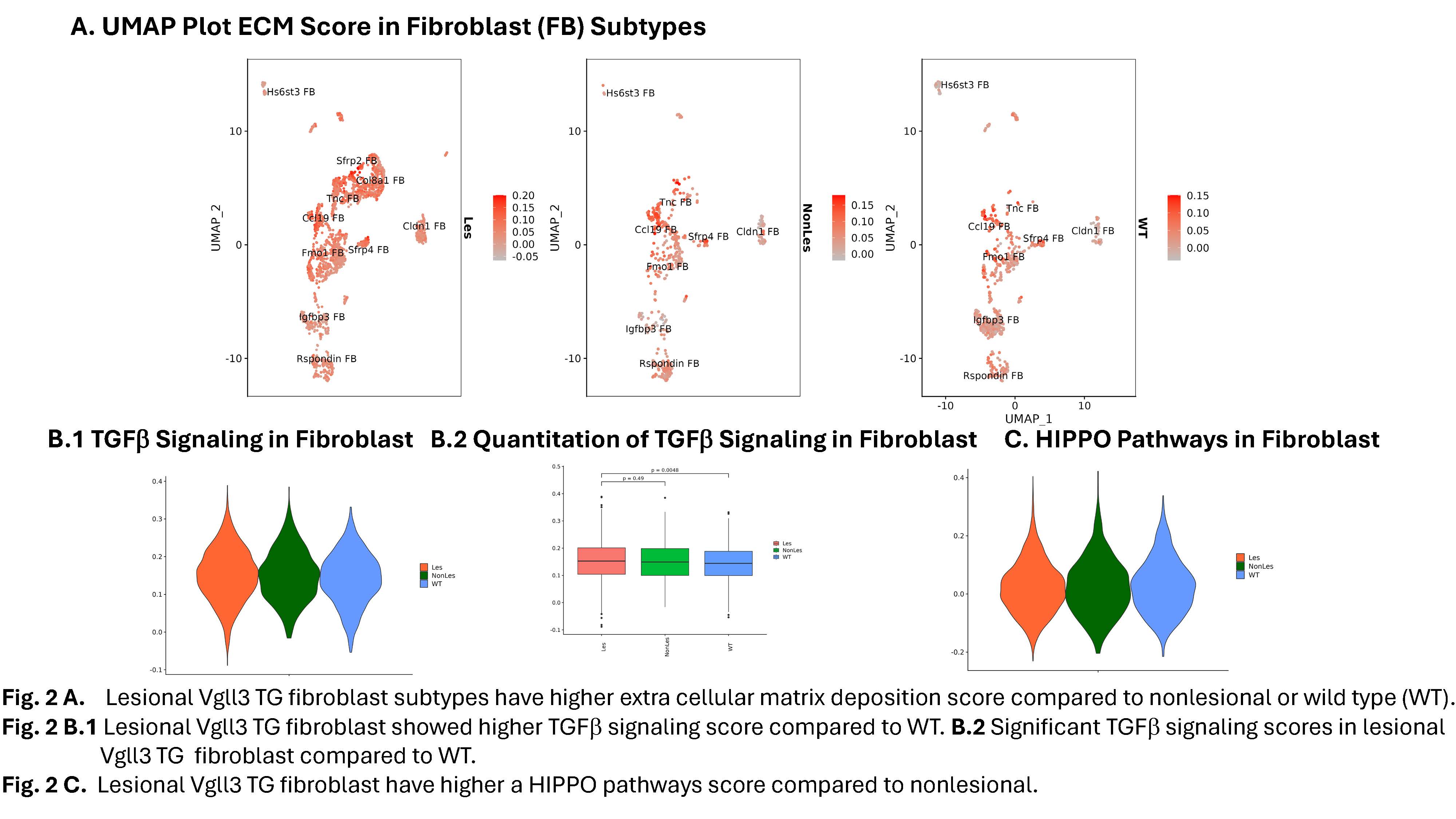
Results: Male and female Vgll3-TG mice exhibit cutaneous inflammation and fibrosis as evidenced by histopathology, trichrome staining, and increased detection of fibrotic markers (ACTA2, COL1A1, COL1A2, TGFB1, CTGF) and pro-fibrotic cytokines. Similar high expression of these fibrotic markers was detected in human DLE lesions. ScRNA-seq of lesional and nonlesional skin from Vgll3-TG vs WT skin showed increased inflammatory infiltrate, which was verified by immunohistochemistry. Myeloid cells, inflammatory gene expression (Nfkb1, Cxcr4, Cxcl2, Il1b, Tnf, Cd14), and Foxp3 and Tgfb1-expressing CD4+ T cells were increased in lesional Vgll3-TG skin. Vgll3-TG skin exhibited higher overall expression of Tgfb1, Col1a1, and Col1a2 compared to nonlesional TG and WT mouse skin (transcript and protein). Further, we identified several subclusters of myofibroblasts, Col8a1+ fibroblast, Sfrp2+ fibroblast, and specific pro-fibrotic Tnc+ fibroblasts in the lesional Vgll3-TG skin (Fig.1). The fibrotic fibroblasts and myofibroblasts expressed inflammatory and fibrotic mediators and extracellular matrix (Fig.2A). Significant increased expression of TGFb and Hippo pathway-regulated genes was seen in fibro/myofibroblast (Fig.2B-C) and keratinocytes from lesional and nonlesional TG skin, suggesting Hippo target dysregulation is an early event in fibrosis driven by epidermal Vgll3 that may drive pathologic myofibroblast differentiation.
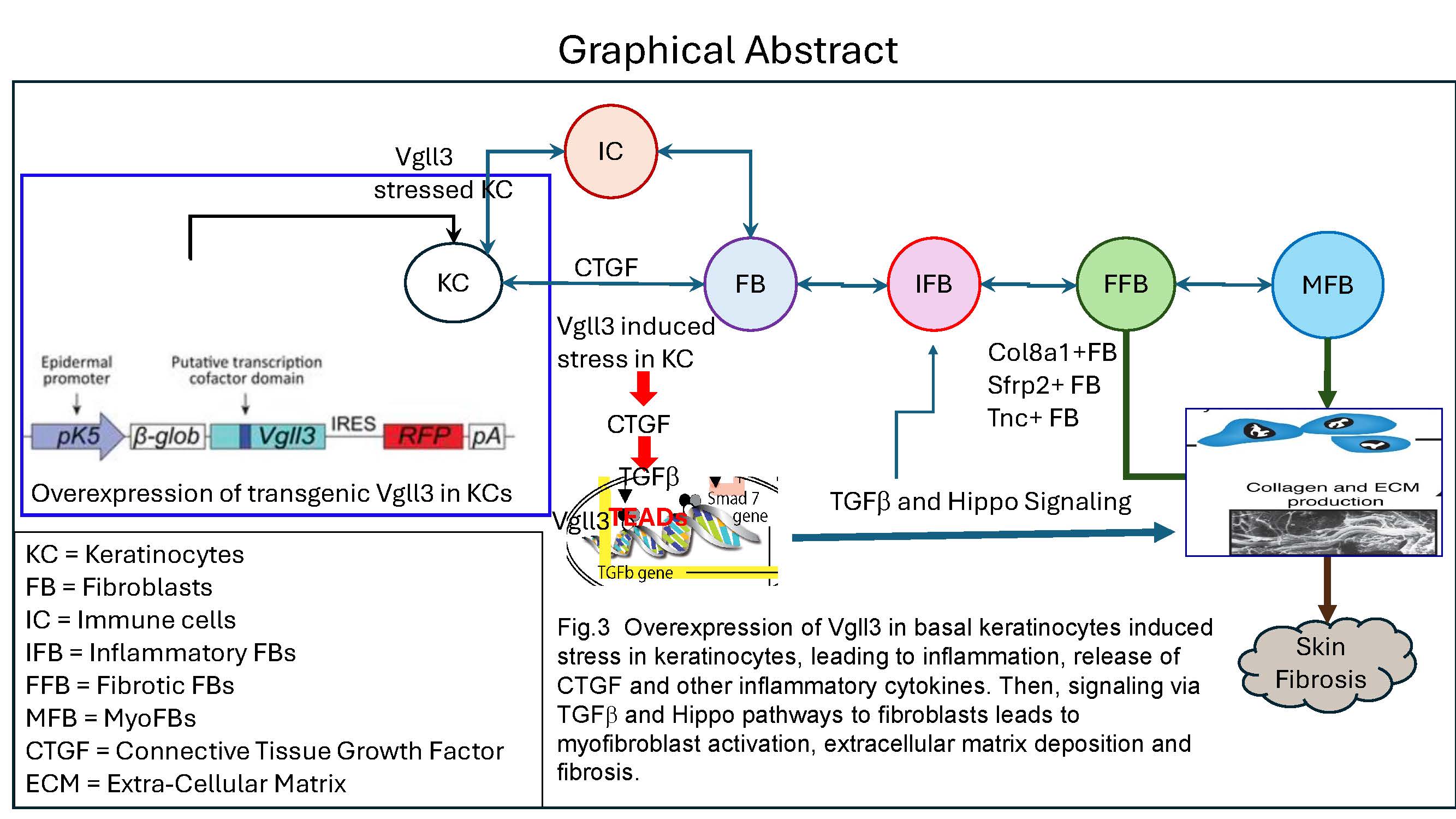
Conclusion: These data are consistent with a model wherein Vgll3-stressed keratinocytes in TG mice release CTGF and other inflammatory cytokines that signal to FB via TGFb and Hippo pathways, leading to myofibroblast activation, extracellular matrix deposition, and fibrosis (Fig.3). These data have important implications for novel therapeutic development for DLE and other fibrosing skin disorders.
Fig. 2 B.1 Lesional Vgll3 TG fibroblast showed higher TGFb signaling score compared to WT. B.2 Significant TGFb signaling scores in lesional
Vgll3 TG fibroblast compared to WT.
Fig. 2 C. Lesional Vgll3 TG fibroblast have higher a HIPPO pathways score compared to nonlesional.
To cite this abstract in AMA style:
Gharaee-Kermani M, Billi A, Dey P, Martens J, Hildebrandt M, Kahlenberg J, Gudjonsson J. Single-Cell RNA Sequencing Outlines the Mechanisms of Vgll3-Driven Myofibroblasts Differentiation in Promoting Fibrosis in Cutaneous Lupus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/single-cell-rna-sequencing-outlines-the-mechanisms-of-vgll3-driven-myofibroblasts-differentiation-in-promoting-fibrosis-in-cutaneous-lupus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-rna-sequencing-outlines-the-mechanisms-of-vgll3-driven-myofibroblasts-differentiation-in-promoting-fibrosis-in-cutaneous-lupus/