Session Information
Session Type: Abstract Session
Session Time: 10:30AM-10:45AM
Background/Purpose: Synovial fibroblasts are key inflammatory aggressors in rheumatoid arthritis (RA) that mediate cartilage and bone destruction, yet therapies directly targeting these cells are lacking. Our previous single cell analyses revealed multiple clusters including a fibroblast subpopulation characterized by high expression of DKK3, a Wnt-associated gene. Wnt activation can promote cell proliferation, invasion, and differentiation, making it an attractive, potentially targetable pathway in RA. Here, we report that non-canonical Wnt signaling drives a strong inflammatory gene expression signature among synovial fibroblasts.
Methods: Bulk and single cell RNA sequencing data from the Accelerating Medicines Partnership (AMP) RA/SLE Network were used to analyze expression of Wnt pathway members among synovial populations in osteoarthritis (OA) and RA. To delineate fibroblast-specific patterns of Wnt signaling, we further utilized a comprehensive stromal cell-selected single cell dataset from the Roche Fibroblast Network Consortium that includes over 73,000 fibroblasts derived from OA and RA synovium as well as other chronic inflammatory diseases. Trajectory analysis was employed to identify a gradient of Wnt expression across fibroblast populations. We developed Wnt activation signatures using in vitro stimulation of human RA-derived synovial fibroblast lines treated with canonical and non-canonical Wnt ligands.
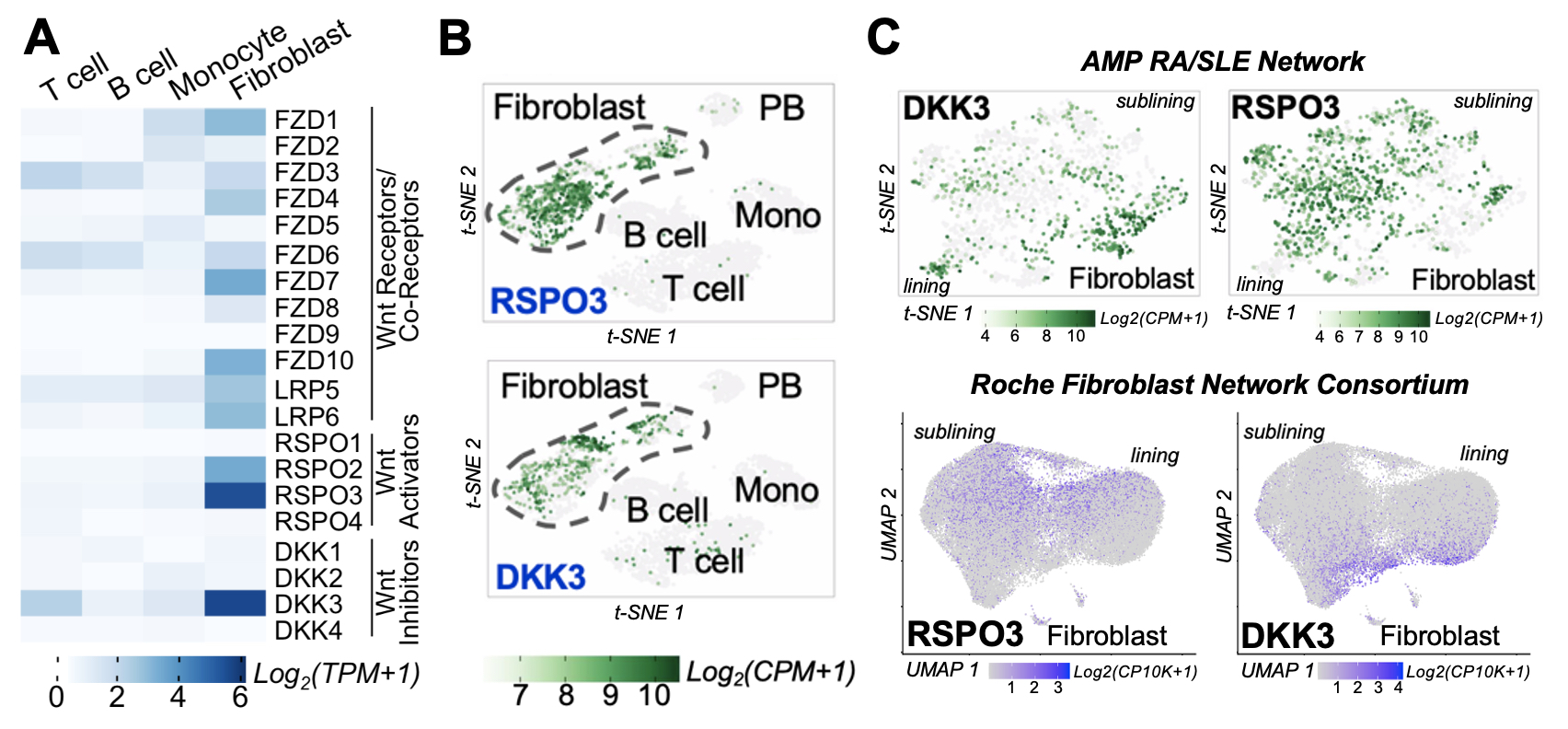
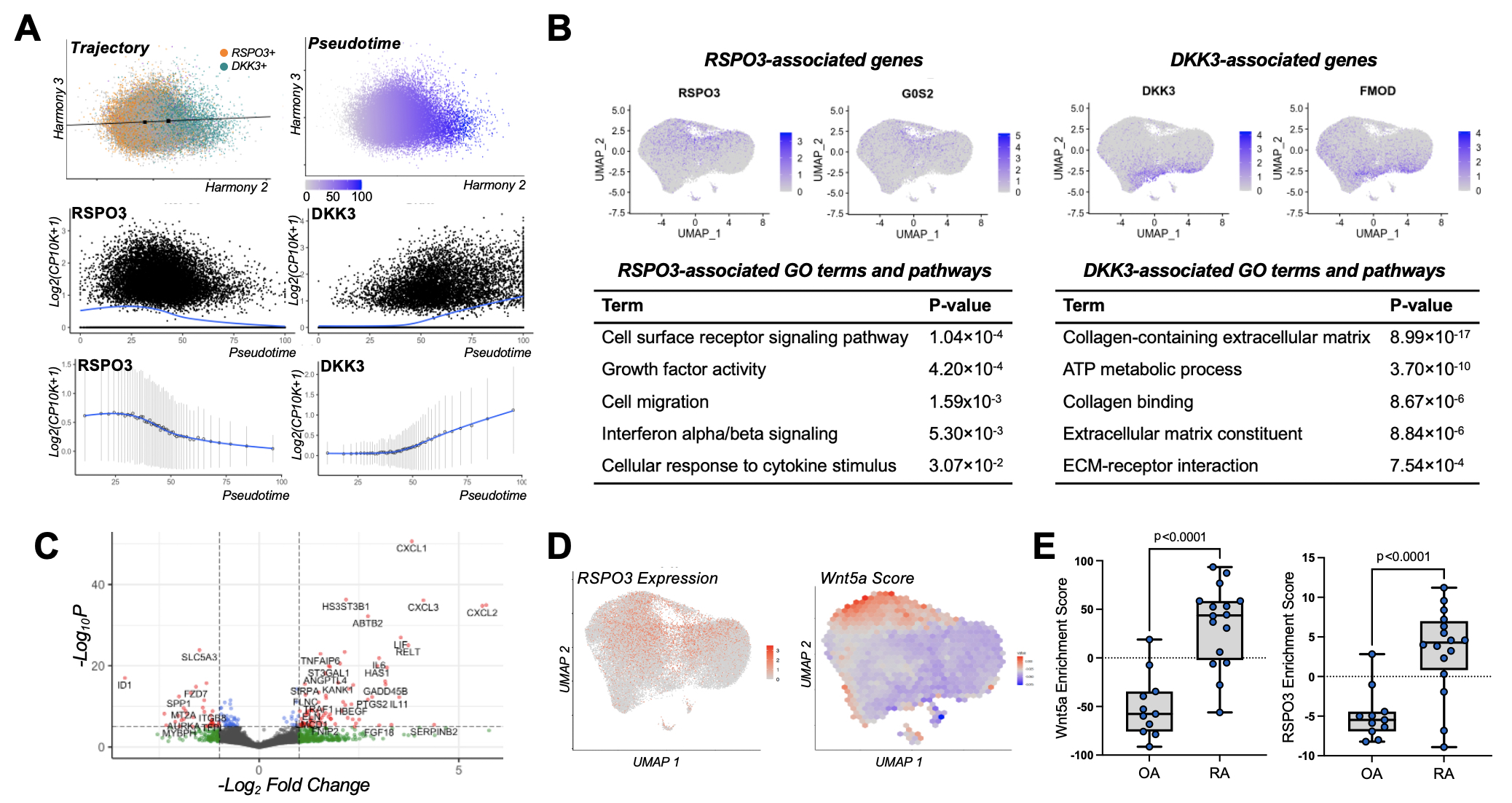
Results: Among all synovial cell populations, Wnt pathway members are predominantly expressed in fibroblast subsets. These molecules include Frizzed receptors, LRP5/6 co-receptors, R-spondin (RSPO) Wnt agonists, and Dickkopf (DKK) Wnt antagonists. Interestingly, non-canonical Wnt ligands dominate in expression as do RSPO3 and DKK3 (Figure 1A and 1B). Strikingly, RSPO3 and DKK3 are expressed reciprocally in distinct populations. Instead of being cluster-specific, RSPO3 and DKK3 form a strong gradient along both lining and sublining fibroblasts (Figure 1C). Using trajectory analysis, we demonstrate that RSPO3 and DKK3 correlate with genes that are differentially associated with inflammation and tissue repair, respectively (Figure 2A and 2B). To explore Wnt pathway activity along this gradient, we generated a non-canonical Wnt activation signature using synovial fibroblasts stimulated with Wnt5a. This signature reveals that non-canonical Wnt signaling drives fibroblast inflammation (Figure 2C) and is particularly active among cells expressing Wnt agonist RSPO3 (Figure 2D). The Wnt5a and RSPO3 gene signatures are also enhanced in fibroblasts derived from inflamed RA synovial tissue compared with those from OA synovial tissue while the DKK3 signature is reduced, suggesting increased Wnt activity in inflammatory RA (Figure 2E). Moreover, analyses show comparable Wnt-mediated gradients in Sjogren’s syndrome and interstitial lung disease, indicating broader disease applicability.
Conclusion: We identify non-canonical Wnt signaling as a novel mechanism contributing to RA synovial fibroblast heterogeneity and inflammatory phenotype. This work provides a foundation for the development of Wnt-modulating pharmacologic strategies targeting synovial fibroblasts in RA.
To cite this abstract in AMA style:
Mueller A, Zou A, Taylor E, Major T, Gardner D, Croft A, Fibroblast Network Consortium R, Filer A, Buckley C, Wei K, Korsunsky I, Raychaudhuri S, Brenner M. Single Cell Profiling Reveals a Wnt-mediated Transcriptional Gradient That Drives Inflammation in Rheumatoid Arthritis Synovial Fibroblast Pathology [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/single-cell-profiling-reveals-a-wnt-mediated-transcriptional-gradient-that-drives-inflammation-in-rheumatoid-arthritis-synovial-fibroblast-pathology/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-profiling-reveals-a-wnt-mediated-transcriptional-gradient-that-drives-inflammation-in-rheumatoid-arthritis-synovial-fibroblast-pathology/