Session Information
Date: Tuesday, October 28, 2025
Title: (1855–1876) Systemic Sclerosis & Related Disorders – Basic Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Antinuclear antibodies (ANA) are detected in over 95% of systemic sclerosis (SSc) patients. Compared to cutaneous subtype classification, autoantibody-based stratification more accurately predicts survival, disease progression, and organ involvement. The distinct clinical features associated with each SSc-specific autoantibody suggest underlying molecular phenotypic differences. Recent studies have revealed distinct molecular signatures between SSc patients with anti-centromere (ACA) and anti-topoisomerase antibodies (ATA). However, the molecular features of those less frequent autoantibody groups have not been studied.
Methods: This study aims to investigate the shared and unique molecular signatures among SSc patients with different SSc-associated autoantibody group (ACA, ATA, anti-RNA polymerase III antibodies (ARA), anti-U3RNP, anti-U1RNP, anti-Th/To, and anti-Ku antibodies) through a multi-omics analysis including plasma proteomics, PBMC transcriptomics, and immune cell phenotyping.
Results: 166 Asian SSc patients were included in this study and grouped by different SSc-associated antibody status: 55 patients with ACA, 58 with ATA, 24 with ARA, 12 with anti-U1RNP, 8 with anti-U3RNP, 4 with anti-Ku, and 5 with anti-Th/To antibodies. Plasma proteomic profiling identified 1,159 proteins, with 846 remaining after stringent filtering. Differential expression analysis revealed 86 consistently upregulated proteins across all antibody subgroups compared to healthy controls (HCs). These proteins were closely associated with endothelial injury, cell-cell adhesion, extracellular matrix deposition, and growth factor binding, reflecting shared pathogenic mechanisms in SSc. Gene Set Enrichment Analysis (GSEA) further identified antibody-specific pathway activation patterns, correlating with distinct clinical manifestations, including calcinosis, renal involvement, cancer, myopathy, and cardiomyopathy. PBMC RNA-seq analysis demonstrated that common upregulated pathways across autoantibody groups (vs. HCs) were associated with immune activation, the ubiquitin-proteasome system, and cytoskeletal regulation. Additionally, GSEA highlighted unique metabolic and immune activation pathways that varied among autoantibody subgroups. Immune cell phenotyping revealed broadly consistent alterations across SSc subgroups, including increased neutrophils and monocytes, decreased lymphocytes and regulatory B (Breg) cells, and elevated activated T cells. However, the magnitude of these changes differed significantly among autoantibody-defined subgroups, indicating heterogeneous immune dysregulation in SSc.
Conclusion: This study provides the first multi-omics characterization of both common and rare SSc-associated autoantibody groups, revealing shared and distinct molecular signatures that correlate with clinical features. Our findings highlight the potential for autoantibody-based stratification to guide precision management of SSc. Future studies should validate these molecular profiles in larger cohorts and explore their therapeutic implications, paving the way for biomarker-driven approaches in SSc care.
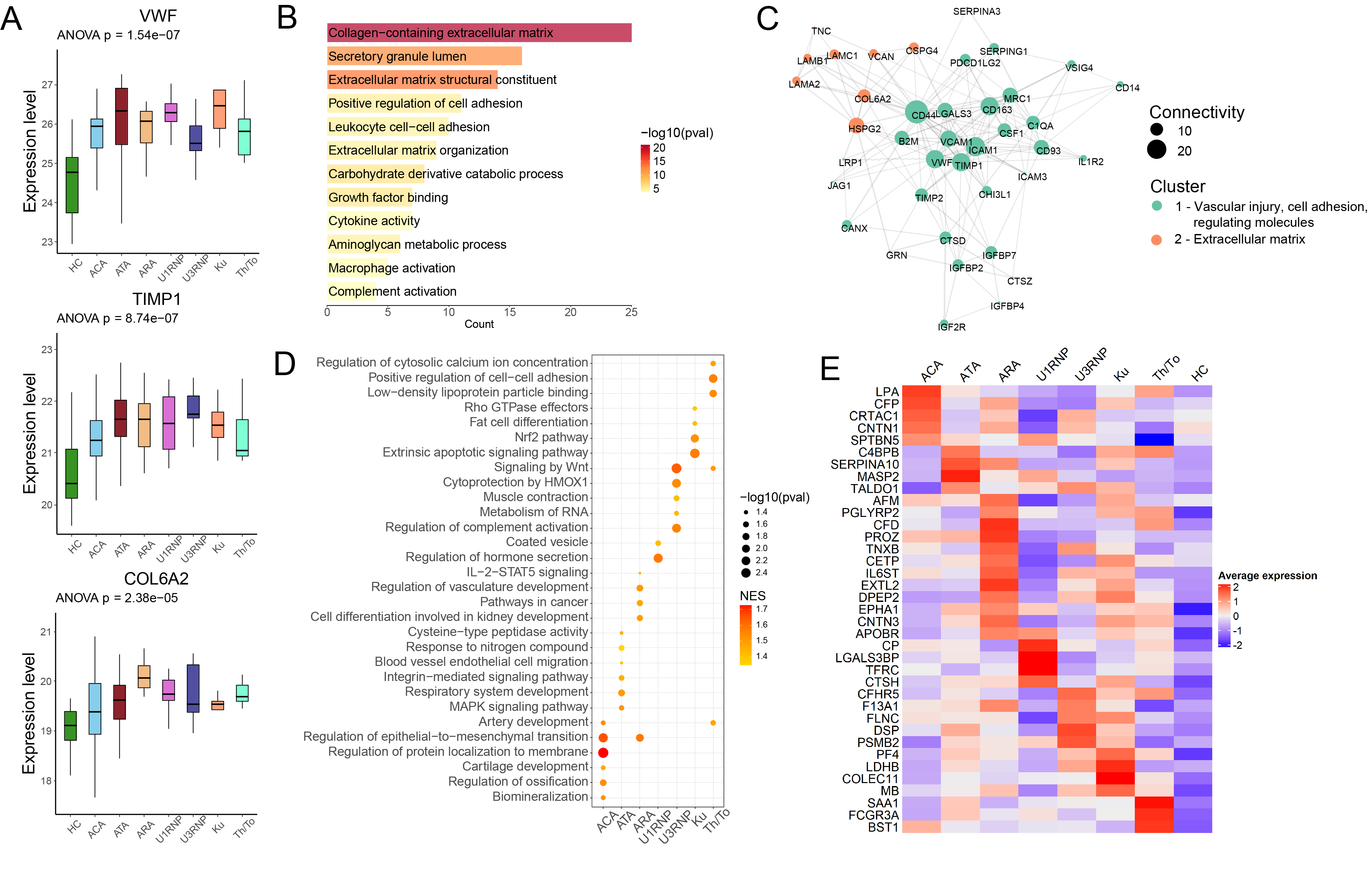
.jpg) Figure 2. Plasma proteomic profiles of different antibody groups compared with HC. (A) Box plots showing log-transformed expression of functionally important shared DEPs. (B) GO enrichment analysis of shared upregulated proteins across antibody groups. (C) Interaction network of shared upregulated proteins with connectivity ≥ 4. The size of the circle represented the degree of each node and the color represented different Louvain clusters. (D) Bubble plot showing uniquely upregulated pathways in different antibody groups identified with GSEA analysis. Pathways with FDR < 0.1 were visualized. The size and the color of the dot denote log-transformed FDR and NES of the corresponding pathways, respectively. (E) Heatmap displaying scaled expression of uniquely upregulated proteins in different antibody groups.
Figure 2. Plasma proteomic profiles of different antibody groups compared with HC. (A) Box plots showing log-transformed expression of functionally important shared DEPs. (B) GO enrichment analysis of shared upregulated proteins across antibody groups. (C) Interaction network of shared upregulated proteins with connectivity ≥ 4. The size of the circle represented the degree of each node and the color represented different Louvain clusters. (D) Bubble plot showing uniquely upregulated pathways in different antibody groups identified with GSEA analysis. Pathways with FDR < 0.1 were visualized. The size and the color of the dot denote log-transformed FDR and NES of the corresponding pathways, respectively. (E) Heatmap displaying scaled expression of uniquely upregulated proteins in different antibody groups.
To cite this abstract in AMA style:
Yin H, Lin W, zhao Z, Jia C, Lu L. Shared and unique molecular signatures across different autoantibody groups in systemic sclerosis: a multi-omics analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/shared-and-unique-molecular-signatures-across-different-autoantibody-groups-in-systemic-sclerosis-a-multi-omics-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/shared-and-unique-molecular-signatures-across-different-autoantibody-groups-in-systemic-sclerosis-a-multi-omics-analysis/