Session Information
Date: Sunday, October 26, 2025
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: Urine collects the byproducts of kidney biology and has emerged as a valuable, noninvasive source of molecular information that reflects intrarenal pathology. In lupus nephritis (LN), multiple urinary signatures have been identified that associate with histologic activity, immune cell infiltration, and response to therapy. However, many of these biomarkers are not disease-specific and may also be elevated in other glomerular conditions. Comparing urinary proteomic profiles across inflammatory and non-inflammatory kidney diseases may uncover shared mechanisms of injury, while also revealing LN-specific signatures that could aid in diagnosis, monitoring, and therapeutic targeting.
Methods: Urine samples from patients with glomerular diseases including LN (ISN classes I–V), ANCA-associated glomerulonephritis, IgA nephropathy (IgAN), membranous nephropathy (MGN), and focal segmental glomerulosclerosis (FSGS) were collected near the time of biopsy and after screening for healthy controls (HC). Proteomic profiles were quantified using the Olink Explore HT platform (5,400 proteins). Here, we report findings for six candidate biomarkers previously linked to LN histological activity and progression: CD163, IL-16, Galectin 1 (LGALS1), Tenascin C (TNC), BAFF (TNFSF13B), and C9.
Results: We analyzed urine samples from 383 patients, including 234 with LN (21 Class I/II, 81 Class III ± V, 70 Class IV ± V, and 62 Class V), 17 with ANCA, 30 with IgAN, 30 with MGN, 30 with FSGS, and 31 healthy controls.IL-16, previously linked to histologically active LN, was elevated in Class III/IV LN and in a subset of ANCA patients. CD163 was elevated in proliferative LN, in a subset of ANCA, and unexpectedly in MGN, but remained low in most IgAN cases . Galectin-1 was broadly elevated across glomerular diseases, but class II LN, IgAN and FSGS had relatively lower levels. TNFSF13B (BAFF) was increased in all conditions, but it was relatively higher in Class III, IV, and V LN, and in MGN, suggesting a stronger role in B cell–driven pathology in these conditions. C9 was elevated across all disease groups, indicating widespread complement activation. Tenascin C was highest in Class IV LN but also elevated in other LN classes and the other glomerular diseases, consistent with activation of prorepair and profibrotic pathways. Complement activation was enriched in all conditions.
Conclusion: Urinary biomarkers reveal both shared and disease-specific molecular pathways across glomerular diseases. The presence of common inflammatory and profibrotic signals—such as complement activation, macrophage involvement, and B cell–driven responses—highlights overlapping mechanisms of injury. At the same time, distinct expression patterns suggest unique pathogenic processes. These findings offer mechanistic insight into glomerular inflammation and fibrosis and, importantly, raise the potential for repurposing targeted therapies across traditionally distinct kidney diseases. Ongoing analysis of the full urinary proteome (~5,400 proteins) is expected to further expand our understanding of disease mechanisms and therapeutic opportunities.
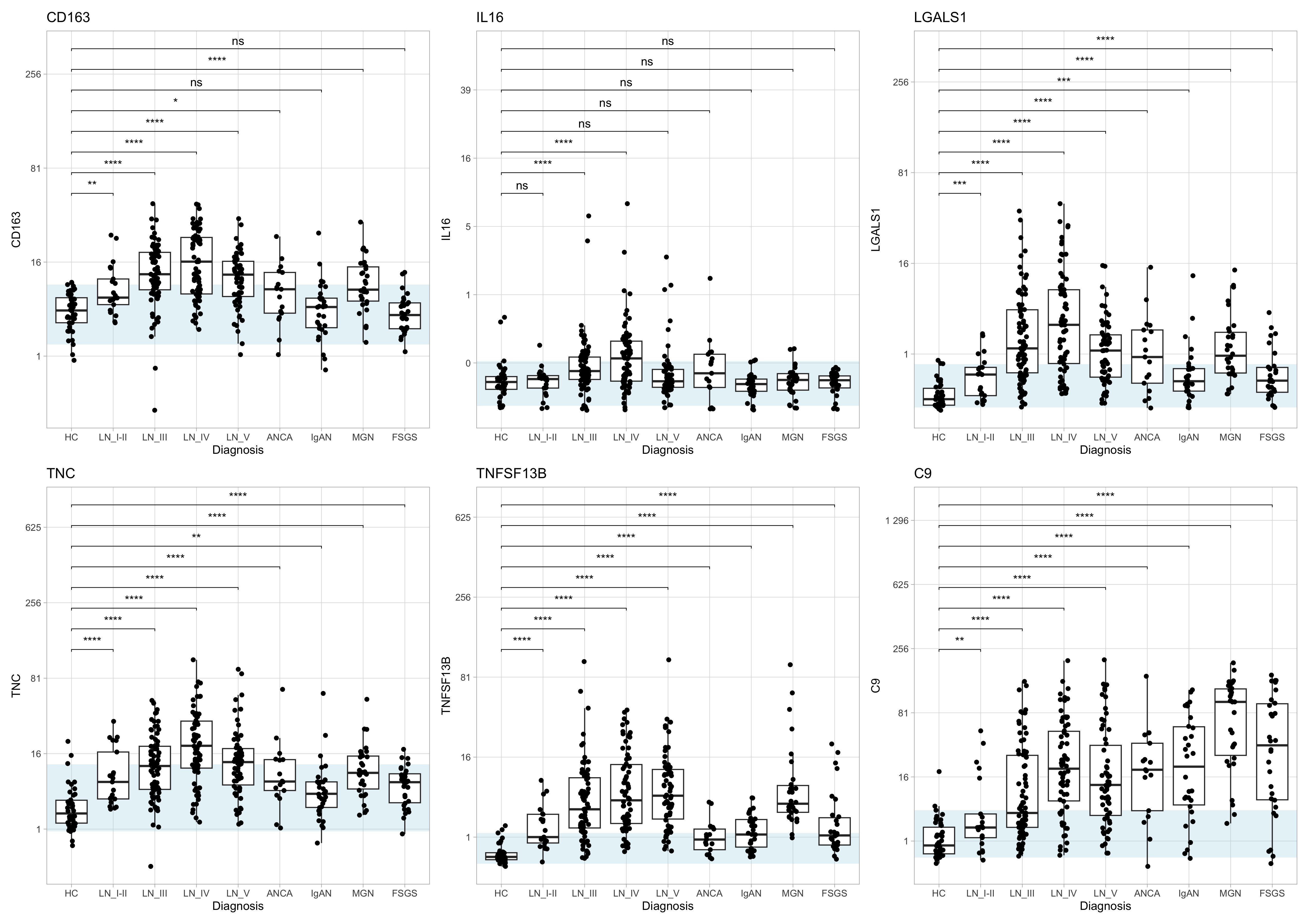 Figure 1. Urinary expression of inflammation- and fibrosis-associated proteins across glomerular diseases. Boxplots show normalized protein expression (NPX) of six urinary biomarkers—CD163, IL-16, Galectin-1 (LGALS1), Tenascin-C (TNC), BAFF (TNFSF13B), and C9—in healthy controls (HC) and patients with lupus nephritis (LN I–V), ANCA-associated vasculitis (ANCA), IgA nephropathy (IgAN), membranous nephropathy (MGN), or focal segmental glomerulosclerosis (FSGS). Each box depicts the median and interquartile range. The light-blue band marks the 5th–95th percentile range of HC values. Wilcoxon rank-sum tests comparing each disease group to HC are shown above each panel (ns, not significant; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
Figure 1. Urinary expression of inflammation- and fibrosis-associated proteins across glomerular diseases. Boxplots show normalized protein expression (NPX) of six urinary biomarkers—CD163, IL-16, Galectin-1 (LGALS1), Tenascin-C (TNC), BAFF (TNFSF13B), and C9—in healthy controls (HC) and patients with lupus nephritis (LN I–V), ANCA-associated vasculitis (ANCA), IgA nephropathy (IgAN), membranous nephropathy (MGN), or focal segmental glomerulosclerosis (FSGS). Each box depicts the median and interquartile range. The light-blue band marks the 5th–95th percentile range of HC values. Wilcoxon rank-sum tests comparing each disease group to HC are shown above each panel (ns, not significant; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
To cite this abstract in AMA style:
Celia A, Saksena D, LEE C, Guthridge C, DeJager W, Lu R, James J, Buyon J, Petri M, Guthridge J, Rovin B, Fava A. Shared and Distinct Urinary Proteomic Signatures of Lupus Nephritis and Other Glomerular Diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/shared-and-distinct-urinary-proteomic-signatures-of-lupus-nephritis-and-other-glomerular-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/shared-and-distinct-urinary-proteomic-signatures-of-lupus-nephritis-and-other-glomerular-diseases/
