Session Information
Date: Tuesday, October 28, 2025
Title: (1780–1808) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteoarthritis (OA) is a chronic, debilitating joint disease affecting 595 million people worldwide, including over 32 million in the United States. It is characterized by progressive degeneration of articular cartilage, most commonly in the knees, hips, and hands. Risk increases with age, joint overuse, and obesity. Despite an annual economic burden of $550 billion in the US, no FDA approved disease-modifying osteoarthritis drugs (DMOADs) are currently available. In this study, we evaluate the therapeutic potential of SEV-101, a novel exosome-based treatment, to promote hyaline cartilage regeneration in weight-bearing regions of the knee in a rat model of OA.
Methods: SEV-101 is a novel exosome therapeutic isolated from induced pluripotent stem cell (iPSC)-derived cartilage tissue. To evaluate the efficacy of SEV-101 in regenerating hyaline cartilage, the rat monosodium iodoacetate (MIA) model of OA was utilized. On Day 1, animals received a unilateral intra-articular injection of MIA in the knee (femorotibial) joint. Treatment began on Day 4 and was administered once per week for 10 weeks. Animals were randomized into four treatment groups (7 males, 7 females per group) and one naïve control group (5 males, 5 females). SEV-101 was dosed at 2.2 × 10¹¹ exosomes. Phosphate-buffered saline served as a vehicle control. FGF-18 was used as a positive control as it has been shown to promote cartilage regeneration. A standard-of-care positive control for pain included intra-articular triamcinolone and oral tramadol. In-life assessments included safety monitoring and Dynamic Weight Bearing (DWB) analysis. Animals were sacrificed on Day 72, and joints underwent histological evaluation, including quantitative analysis of hyaline cartilage regeneration in the medial joint compartment. Rigorous criteria included presence of tidemark and bone resorption, in addition to other measurements that ensured that the cartilage being measured was new, good quality hyaline cartilage.
Results: No safety signals were observed in SEV-101 treated rats. SEV-101 treated male rats showed a statistically significant increase in regeneration of hyaline articular cartilage in weight-bearing regions of the knee joint (designated as “good quality hyaline cartilage”) compared to all other treatment groups. In addition, SEV-101 treated male rats showed more intact meniscus structure and more Type 2 Collagen immunohistochemical staining compared to vehicle controls. There was no significant difference in DWB vehicle controls and SEV-101 treated animals.
Conclusion: SEV-101 promoted the regeneration of new hyaline cartilage rather than merely preserving existing cartilage. Additionally, SEV-101-treated animals exhibited improved meniscal integrity and increased Type II Collagen staining relative to vehicle controls. These findings support SEV-101’s capacity to regenerate cartilage in weight-bearing regions of the joint and to confer structural protection, reducing the risk of further degeneration. Collectively, these data strongly support the continued development of SEV-101 as a DMOAD.
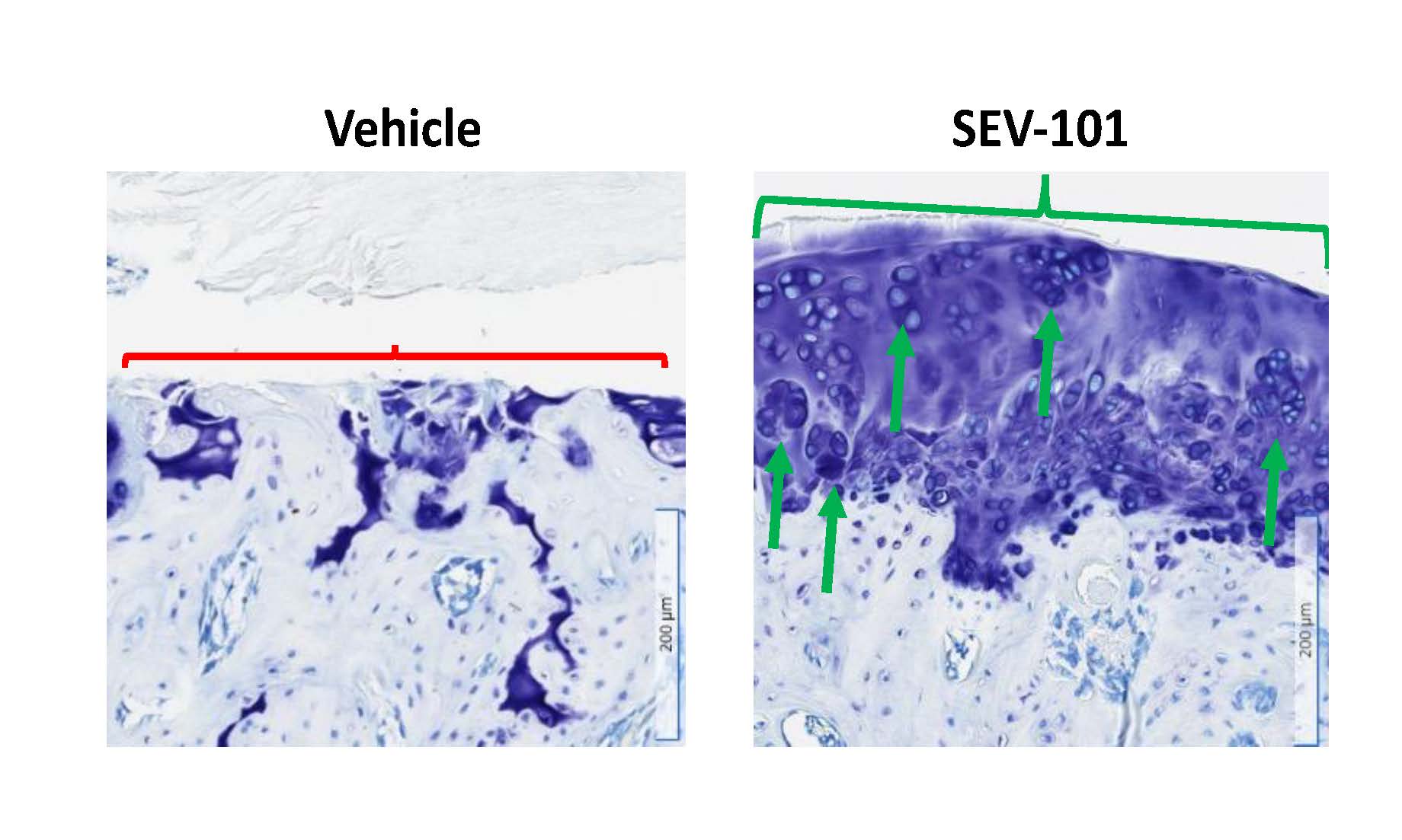 Figure 1. Good Quality Hyaline Cartilage Repair Width in the Medial (weight bearing) Knee Joint. Toluidine Blue Stained Histological sections of knee joints in male animals were assessed using the following criteria to ensure that regeneration, and not preservation was being measured: 1) the tidemark and corresponding bone/calcified cartilage had evidence of resorption, 2) the presence of proteoglycan (any degree of blue pigment) and/or chondrocytes within the intact pre-existing cartilage matrix, 3) had evidence of normal chondrocyte arrangement/maturation (does not have obvious features of fibrocartilage, such as elongate cellular morphology or streaming).
Figure 1. Good Quality Hyaline Cartilage Repair Width in the Medial (weight bearing) Knee Joint. Toluidine Blue Stained Histological sections of knee joints in male animals were assessed using the following criteria to ensure that regeneration, and not preservation was being measured: 1) the tidemark and corresponding bone/calcified cartilage had evidence of resorption, 2) the presence of proteoglycan (any degree of blue pigment) and/or chondrocytes within the intact pre-existing cartilage matrix, 3) had evidence of normal chondrocyte arrangement/maturation (does not have obvious features of fibrocartilage, such as elongate cellular morphology or streaming).
.jpg) Figure 2. Histopathology of the medial compartment of male right stifle (knee) joints stained with toluidine blue. Vehicle control animals showed extensive loss of articular cartilage (red bracket delineates affected areas) with minimal regeneration of articular cartilage evident. SEV-101 treated animals showed prominent regions of articular hyaline cartilage regeneration (green bracket delineates this region and green arrows indicate chondrogenic clusters forming hyaline cartilage).
Figure 2. Histopathology of the medial compartment of male right stifle (knee) joints stained with toluidine blue. Vehicle control animals showed extensive loss of articular cartilage (red bracket delineates affected areas) with minimal regeneration of articular cartilage evident. SEV-101 treated animals showed prominent regions of articular hyaline cartilage regeneration (green bracket delineates this region and green arrows indicate chondrogenic clusters forming hyaline cartilage).
To cite this abstract in AMA style:
Lindborg B, Vegoe A, Williams M, Rangarajan P, Tompkins M, Ramachandran S, O'Brien T. SEV-101, A Regenerative Exosome Therapeutic for the Treatment of Osteoarthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/sev-101-a-regenerative-exosome-therapeutic-for-the-treatment-of-osteoarthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sev-101-a-regenerative-exosome-therapeutic-for-the-treatment-of-osteoarthritis/
