Session Information
Date: Tuesday, November 9, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III (1836–1861)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Limited cutaneous systemic sclerosis (lcSSc) carries a highly variable prognosis and to date there are no stratification tools to predict clinical outcomes. Evidence suggests that type-I Interferon (IFN) pathway is involved in progression of clinical manifestations of diffuse cutaneous SSc (dcSSc). However, the role of type-I IFNs in lcSSc has not yet been explored. Here we set out to determine in the context of an observational study the value of serum Type I IFN activation in stratifying for clinical outcome in lcSSc using a novel composite score developed for the MINIMISE clinical trial of mycophenolate mofetil in lcSSc (EudraCT: 2019-004139-21)
Methods: Consecutive patients with lcSSc (according to LeRoy) were selected within our observational cohort. Serum IFN score was measured as the average of the logged concentration of CCL2, CCL8, CCL19, CXCL9, CXCL10, and CXCL11, assessed by Luminex assay. 38 healthy volunteers were assayed as controls (HC). The composite morbi-mortality endpoint capturing “meaningful disease progression” included (i) New lung fibrosis on HRCT (FVC< 70%), (ii) Deterioration of established lung fibrosis, (iii) Significant progression of skin score, (iv) New major cardiac complication, (v) Scleroderma renal crisis, (vi) new RHC diagnosis of pulmonary hypertension, (vii) Severe SSc related GI disease, (viii) Severe digital vasculopathy and (ix) Mortality. Baseline observation serum from patients was assessed for IFN score. The statistical methodology employed included Pearson/Spearman correlation analysis and Fisher exact test as appropriate. Kaplan Meier curves compared event-free survival.
Results: HC had a mean IFN Score of 4.7 +/- 0.3 STDV. 66 lcSSC patients (64 F, mean age 65.5 years) were included in the analysis. Median(IQR) disease duration was 12 years (5 to 16), with 68 months median Follow up (33 to 88). 33 (50%) patients met the morbi-mortality endpoint within the follow-up period with a mean (STDV) time to event of 43 (26) months. Forty-two (63.63%) patients had anticentromere antibodies of which 35 had no other autoantibody detected. IFN score in lcSSC was not normally distributed. Median (IQR) IFN score of lcSSc patients was significantly higher than HC (5.2 (5,5.4), p< 0.0001). Within the patient cohort, median (IQR) IFN score of patients carrying only ACA was significantly lower than others (5.1 (4.9,5.3) vs 5.4 (5,5.7), p=0.003)). 49 (74%) patients had IFN score higher than Mean+ STDV of HC (IFN HI). 17 (35%) of the IFN HI patients met the morbi-mortality endpoint within 36 months with a mean (STDV) time to endpoint of 40 (23) months, vs only 2 (12%) IFN LO patients (mean time to event 57 months (23))(One-Sided Chi-Square =0.036).
Conclusion: Within the limitations of retrospective analysis of observational cohort, our data suggest that patients with lcSSc do show evidence of Type I IFN dysregulation. Patients with HI Serum IFN scores tend to have worse outcomes than patients with normal or low IFN scores. Once confirmed in additional cohorts, IFN score could be a valuable tool for stratification of lcSSc and may help to better integrate clinical management of limited and diffuse subsets of SSc and facilitate timely implementation of organ-specific therapy across both subsets.
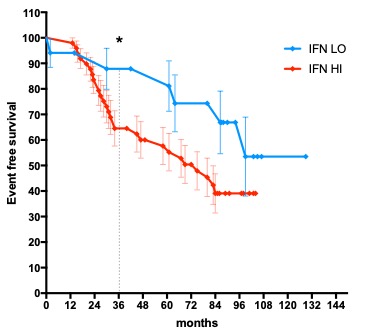 Figure 1. Kaplan Meier curves for event-free survival and difference at 36 months
Figure 1. Kaplan Meier curves for event-free survival and difference at 36 months
To cite this abstract in AMA style:
Karanth R, Abignano G, Kakkar V, Ross R, Denton C, Del Galdo F. Serum IFN Score Predicts Long Term Outcome in Limited Cutaneous SSc [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/serum-ifn-score-predicts-long-term-outcome-in-limited-cutaneous-ssc/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-ifn-score-predicts-long-term-outcome-in-limited-cutaneous-ssc/
