Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
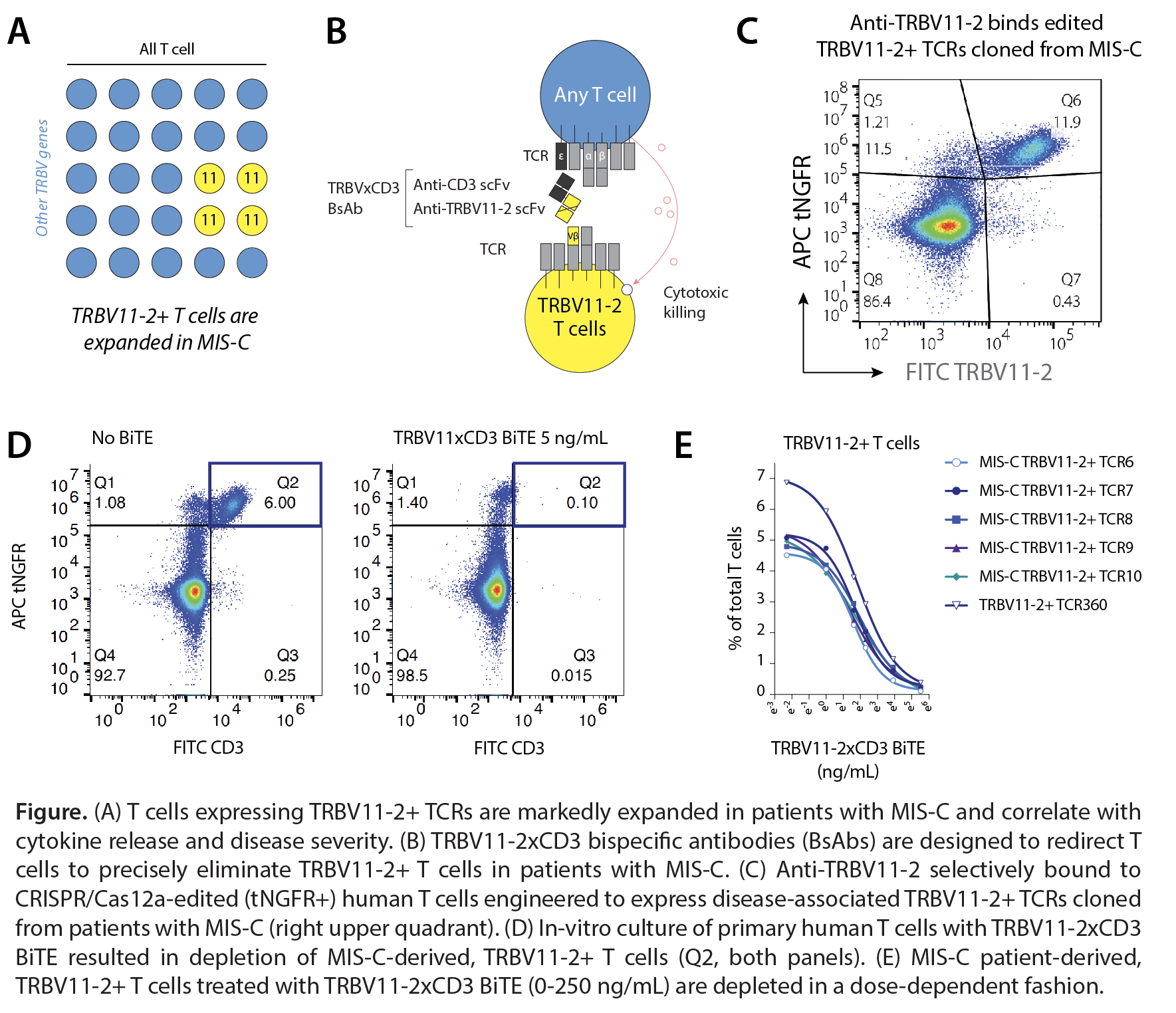
Background/Purpose: Multisystem Inflammatory Syndrome in Children (MIS-C) is a rare but potentially deadly immune complication after infection with SARS-CoV-2. In patients with MIS-C, a striking clonal expansion of T cells using the β-chain variable (TRBV) gene 11-2 (TRBV11-2) is observed and correlates with disease severity and cytokine levels. A superantigen (Sag)-like motif adjacent to the furin-like cleavage site (FCS) in the SARS-CoV-2 spike protein (T678NSPRRARSV687) has been implicated as a potential driver of TRBV11-2+ T cell expansion and hyperinflammation. The treatment for MIS-C involves global immunosuppression, but leaves children vulnerable to infections. Ideal treatments for MIS-C would selectively and rapidly eliminate disease-causing, clonally expanding T cells while sparing protective immune responses. Targeting these T cells through their shared germline encoded regions of the T cell receptor (TCR) provides an opportunity to selectively deplete expanded, TRBV11-2+ T cells. We therefore developed a bispecific T cell engaging antibody (BiTE) therapy that eliminates TRBV11-2+ T cells.
Methods: Anti-TRBV11-2 and anti-CD3 single-chain variable fragment (scFv) sequences were synthesized and cloned into mammalian expression vectors. TRBV11-2xCD3 bispecific T-cell engagers (BiTEs) were expressed in ExpiCHO cells. Polyclonal human T cells were CRISPR-Cas12a-edited to remove or replace their endogenous TCRs with 5 disease-associated TRBV11-2+ TCR sequences cloned from patients with MIS-C. Binding of anti-TRBV11-2 to engineered TRBV11-2+ T cells and T cells expressing other TRBV genes was determined by flow cytometry. Engineered MIS-C-derived TRBV11-2+ T cells were stained with CellTrace Violet, incubated with purified spike protein trimers (with or without FCS mutation) or CD3/CD28 T cell activator (positive control), and proliferation measured by flow cytometry. Engineered MIS-C-derived TRBV11-2+ T cells and polyclonal human T cells were treated with TRBV11-2xCD3 BiTEs and depletion of TRBV11-2+ T cells quantified by flow cytometry.
Results: We developed BiTEs to redirect T cells to selectively eliminate TRBV11-2+ T cells that are associated with hyperinflammation in MIS-C (A-B). Anti-TRBV11-2 selectively bound CRISPR-engineered human T cells expressing disease-associated TRBV11-2+ TCRs cloned from patients with MIS-C (C), but not T cells expressing other TRBV alleles or no TCR. Incubation of engineered MIS-C-derived TRBV11-2+ T cells with intact or FCS-mutant spike protein trimers did not result in TRBV11-2+ T-cell proliferation above background, suggesting that the SAg motif alone is insufficient to explain the clonal expansion of TRBV11-2+ T cells in MIS-C. In culture of engineered and polyclonal T cells, TRBV11-2xCD3 BiTEs selectively depleted TRBV11-2+ T cells in a dose-dependent manner (D).
Conclusion: We describe an off-the-shelf, precision immunotherapy approach for the fast and deep depletion of TRBV11-2+ T cells in patients with MIS-C. TRBV11-2xCD3 BiTEs are highly potent and specific at eliminating TRBV11-2+ T cells. TRBV11-2xCD3 BiTEs highlight the opportunities for TCR allele-targeted precision drugs for the treatment of autoimmune and T cell-mediated diseases.
To cite this abstract in AMA style:
Shaw E, Glavaris S, Mog B, Pearlman A, DiNapoli S, Liu J, Kaeo K, Kinzler K, Bettegowda C, Zhou S, Vogelstein B, Paul S, Konig M. Selectively Targeting TRBV11-2+ T Cells in Multisystem Inflammatory Syndrome in Children (MIS-C) Using Bispecific T Cell-Engaging Antibodies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/selectively-targeting-trbv11-2-t-cells-in-multisystem-inflammatory-syndrome-in-children-mis-c-using-bispecific-t-cell-engaging-antibodies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/selectively-targeting-trbv11-2-t-cells-in-multisystem-inflammatory-syndrome-in-children-mis-c-using-bispecific-t-cell-engaging-antibodies/