Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Mechanistic target of rapamycin (mTOR) inhibitors are effective in animal models of granulomatous disease, but their benefit in patients with sarcoidosis is unknown. We evaluated the incidence of sarcoidosis in patients treated with mTOR inhibitors versus calcineurin inhibitors.
Methods: This was a cohort study using the Optum Clinformatics® Data Mart Database (2003-2019), IBM® MarketScan® Research Database (2006-2016), and Danish health and administrative registries (1996-2018). Patients aged ≥18 years with ≥1 year continuous enrollment before and after a kidney, liver, heart, or lung transplant treated with an mTOR inhibitor or calcineurin inhibitor were included. Patients diagnosed with sarcoidosis before, or up to 90 days after, transplant were excluded. Incidence rates (IRs) were calculated and reported as the number of sarcoidosis events per 1,000 person years, and 95% confidence intervals (CIs) were calculated using the Mid-p exact test.
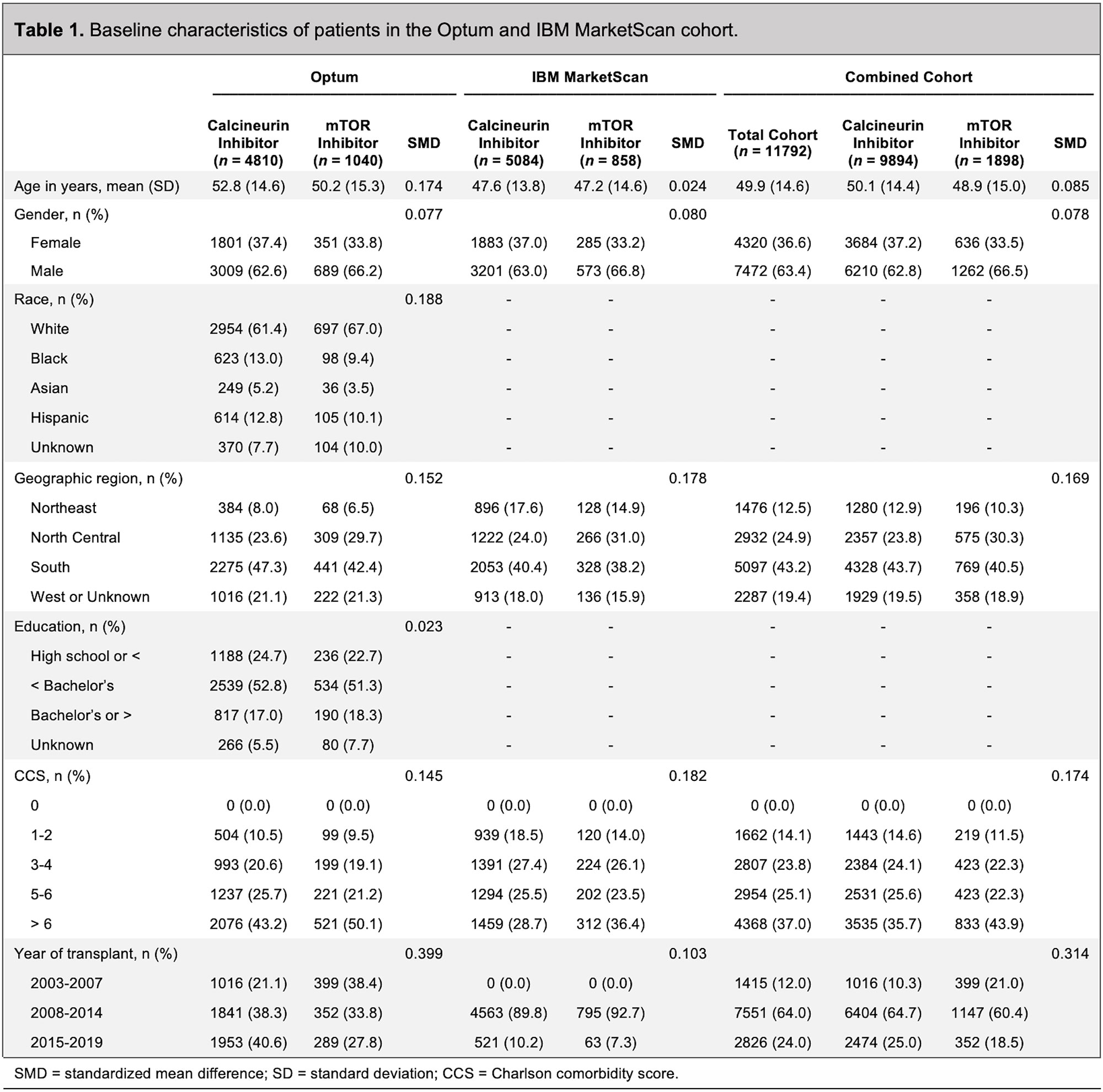
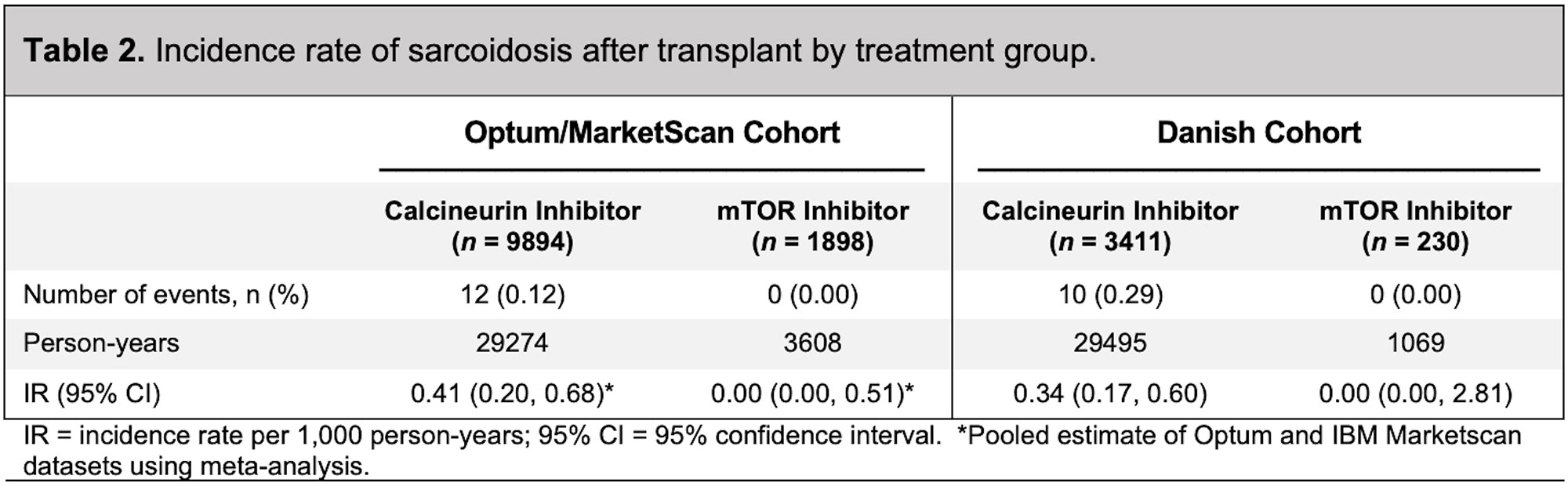
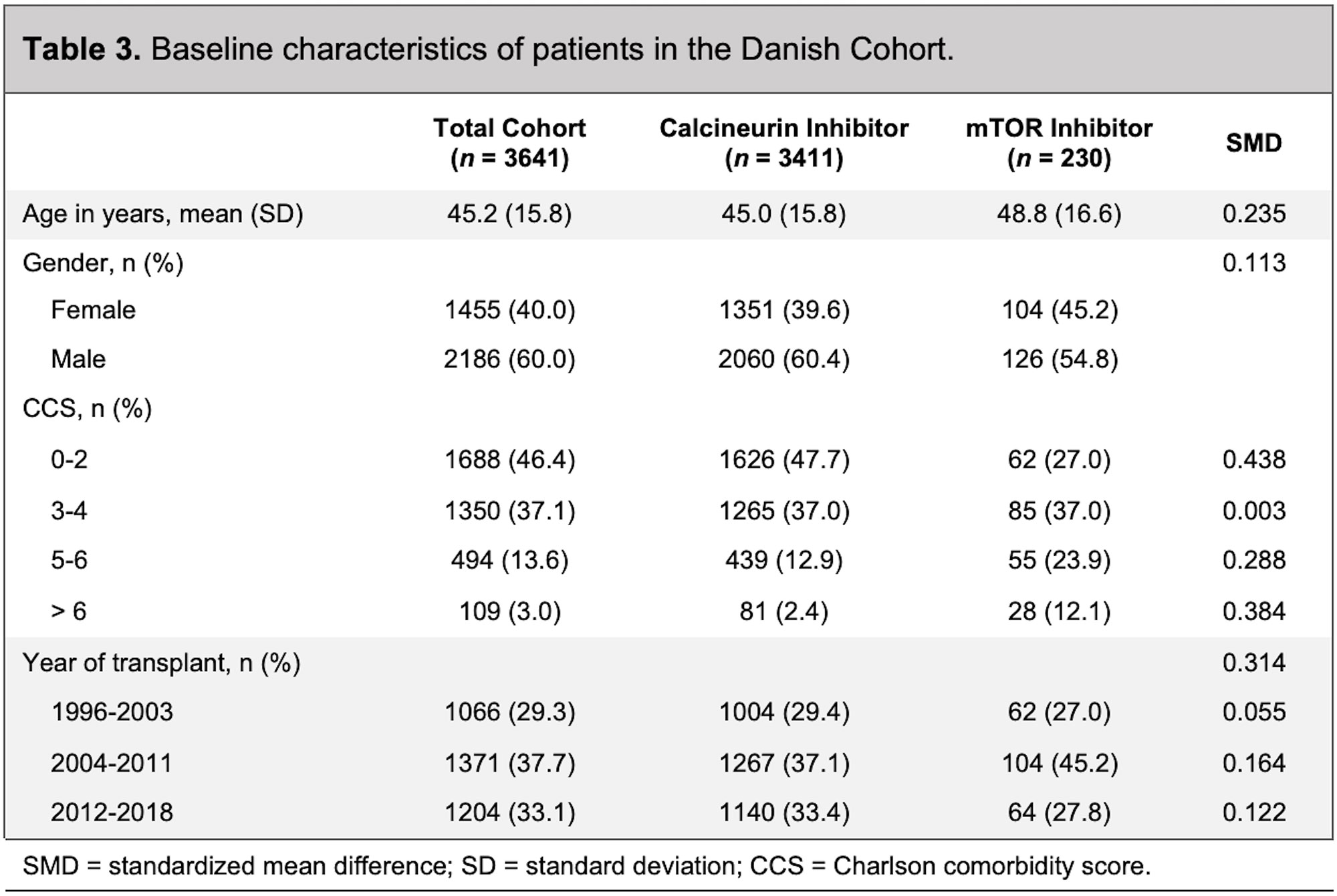
Results: In the Optum/MarketScan cohort, 1,898 patients were treated with an mTOR inhibitor (mean age 49 years; 34% female) and 9,894 patients were treated with a calcineurin inhibitor (mean age 50 years; 37% female) (Table 1). The mean follow-up in the mTOR inhibitor group was 1.1 years, with no incident sarcoidosis diagnosed. In the calcineurin inhibitor group, the mean follow-up was 2.2 years, with 12 incident cases of sarcoidosis diagnosed (Table 2). Patients receiving calcineurin inhibitors were more likely to receive mycophenolate mofetil (85.5% versus 43.5%) and azathioprine (6.3% versus 3.0%) than patients receiving mTOR inhibitors. The median dose of prednisone in the calcineurin inhibitor group was 11.7 mg/day compared with 5.8 mg/day in the mTOR inhibitor group (p< 0.001). In the Danish cohort, 230 patients were treated with an mTOR inhibitor (mean age 49; 45% female), with no incident sarcoidosis diagnosed (Table 3 and Table 2). There were 3,411 patients treated with a calcineurin inhibitor (mean age 45; 40% female), with 10 incident cases of sarcoidosis diagnosed (Table 3 and Table 2). The length of follow-up was shorter in the mTOR inhibitor group (median of 3.3 years) compared with the calcineurin inhibitor group (median of 7.8 years). Patients receiving calcineurin inhibitors were more likely to receive prednisone (47% versus 31%) than patients receiving mTOR inhibitors. The median dose of prednisone was also higher in the calcineurin inhibitor group (2.0 mg/day) compared with the mTOR inhibitor group (1.2 mg/day) (p< 0.685).
Conclusion: We observed a lower incidence of sarcoidosis in solid organ transplant patients receiving mTOR inhibitors compared with patients receiving calcineurin inhibitors. Results from this study further support preclinical data and case reports demonstrating that mTOR inhibitors may provide therapeutic benefit in patients with sarcoidosis. Future interventional studies with mTOR inhibitors for the treatment of sarcoidosis could be considered.
To cite this abstract in AMA style:
Baker M, Vágó E, Liu Y, Lu R, Tamang S, Horváth-Puhó E, Sørensen H. Sarcoidosis Incidence After mTOR Inhibitor Treatment [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/sarcoidosis-incidence-after-mtor-inhibitor-treatment/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sarcoidosis-incidence-after-mtor-inhibitor-treatment/