Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: B-cells and plasma cells are key drivers of systemic autoimmune diseases (AID). Recent data demonstrated preliminary safety and efficacy of using the CD3xCD19 bispecific T-cell engager Blinatumomab in refractory rheumatoid arthritis (1). To target CD19-negative-long-lived plasma cells, BCMA is a highly effective target in multiple myeloma and can be promising new target for AID (2).
Methods: Patients with active, refractory autoimmune diseases including rheumatoid arthritis (RA), dermatomyositis (IIM), Systemic sclerosis (SSc) and primary Sjoegren-Syndrome (SjS) received therapy with bispecific T cell engagers at our center. Patients were treated with CD3xCD19 Blinatumomab or CD3xBCMA Teclistamab in a named patient program. Adverse events including cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS) and immune effector cell–associated hematotoxicity syndrome (ICAHTS), were monitored after standardized screening.
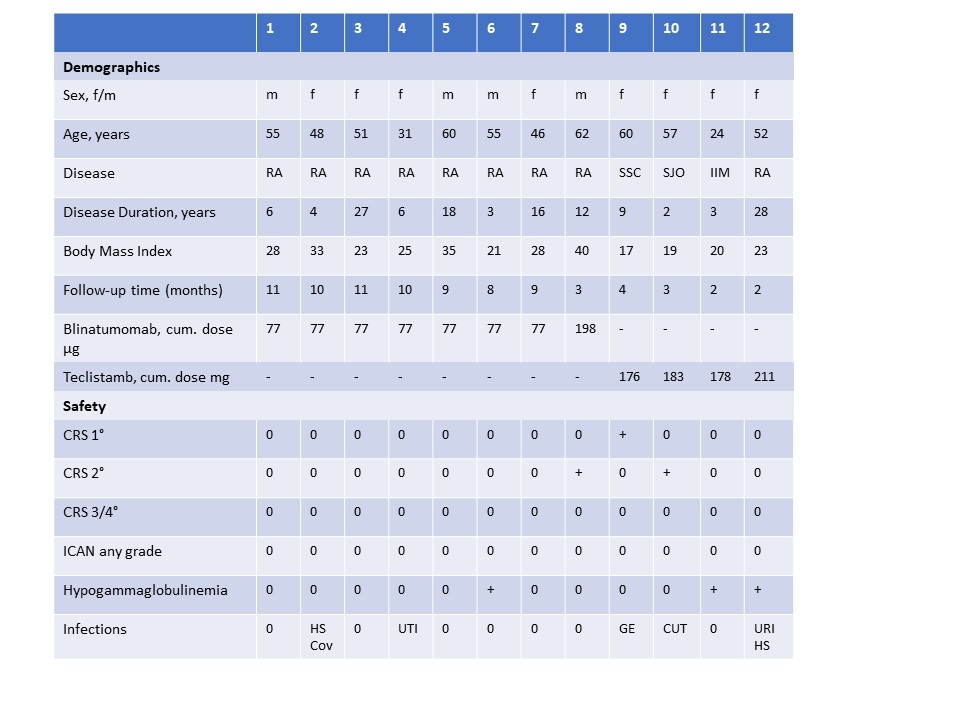
Results: 12 Patients (9 RA, 1 SSc, 1 IIM, 1 SjS) were treated. 8 patients with RA received Blinatumomab (7x cumulative dose 77µg, 1x 198µg) and 4 patients (RA, SSc, IIM, SJO) recevied Teclistamab in a step-up regime according to their body weight. All patients showed active, progressive and multiple therapy refractory disease activity. All patients were followed up longer than 28 days (Table 1). CRS occurred in 3/12 patients, while all CRS were mild (1x grade 1, 2x grade 2) and discontinued after single dose Tocilizumab. ICAN was not oberseved at all. Infections occurred in 5/12 patients (2 Blinatumomab, 3 Teclistamab), all of them mild infections (herpes simplex, SarsCov2, urinary tract infection, gastroenteritis, cutanous folliculitis, upper respiratory infection). Hypogammaglobulinemia were recorded in three patients and substituted with IVIG (1x after Blinatumomab, 2x after Teclistamab). No further myelotxocity was oberserved, neither in long-term follow-up.
Conclusion: Treatment with bispecific T-cell engagers had a favorable safety profile in 12 patients with refractory autoimmune disease. Hence, targeting CD19 or BCMA with T cell engagers appears feasible in autoimmune disease and warrants further clinical development.
References:
1. Bucci L, Hagen M, Rothe T, et al. Bispecific T cell engager therapy for refractory rheumatoid arthritis. Nat Med 2024. DOI: 10.1038/s41591-024-02964-1.
2. Moreau P, Garfall AL, van de Donk N, et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2022;387(6):495-505. DOI: 10.1056/NEJMoa2203478.
To cite this abstract in AMA style:
Hagen M, Bucci L, Boeltz S, Noethling D, Rothe T, Raimondo M, Tur C, Wirsching A, Wacker J, Bozec A, Schett G, Grieshaber-Bouyer R. Safety of Bispecific T-cell Engager Therapy in Autoimmune Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-of-bispecific-t-cell-engager-therapy-in-autoimmune-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-bispecific-t-cell-engager-therapy-in-autoimmune-disease/