Session Information
Date: Sunday, November 12, 2023
Title: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients with refractory gout, the inability to maintain serum uric acid (sUA) levels < 6 mg/dL leads to severe clinical manifestations for which uricase-based therapies can be highly effective, though also immunogenic.1 SEL-212 is a once-monthly, novel 2-component, uricase-based infusion therapy being investigated in patients with refractory gout. SEL-212 consists of an infusion of tolerogenic nanoparticles containing rapamycin (SEL-110) followed by pegadricase (SEL-037).2 DISSOLVE I and II (D1 and D2, respectively) evaluated the safety and efficacy of SEL-212 in adults with refractory gout.
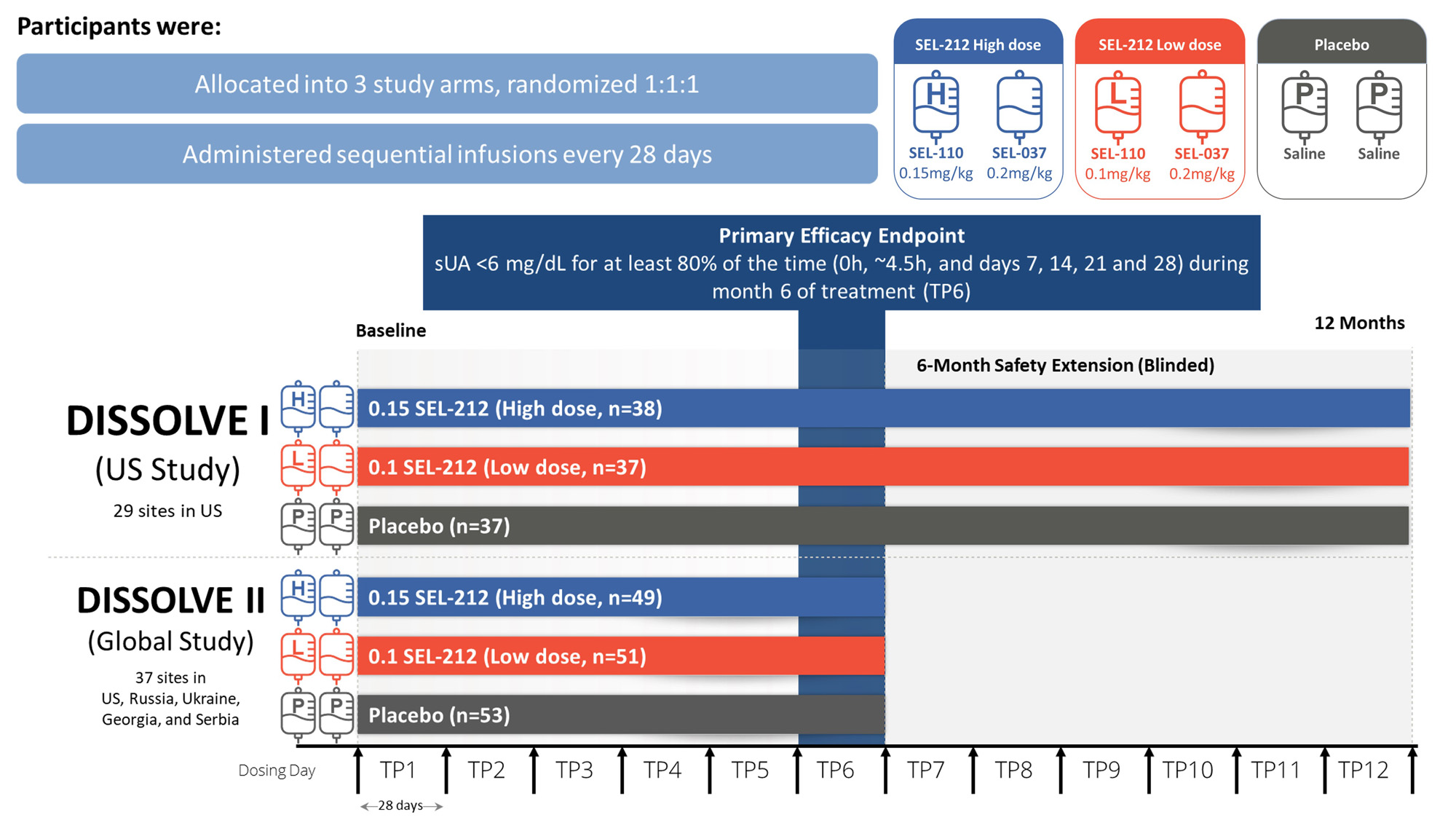
Methods: D1 (US) and D2 (Global), were placebo-controlled, double-blind, randomized clinical trials that evaluated two dose levels of SEL-110 (0.15 mg/kg [high-dose] or 0.1 mg/kg [low-dose]) prior to SEL-037 (0.2 mg/kg) infusion. Participants were enrolled if they had ≥ 3 gout flares within 18 months prior to screening or ≥ 1 tophus or a current diagnosis of gouty arthritis, failed to normalize sUA and control symptoms with any xanthine oxidase inhibitor, and were not previously exposed to a pegylated uricase-based therapy. Participants were randomized 1:1:1 between the two doses of SEL-212 and placebo administered intravenously every 28 days for 6 treatments. D1 participants continued in a 6-month blinded extension phase under the initial treatment conditions (Fig. 1). The primary endpoint was defined as the percentage of participants who achieved and maintained sUA < 6 mg/dL for ≥ 80% of the sixth treatment period (TP6). Safety and tolerability were assessed through monitoring of adverse events (AEs).
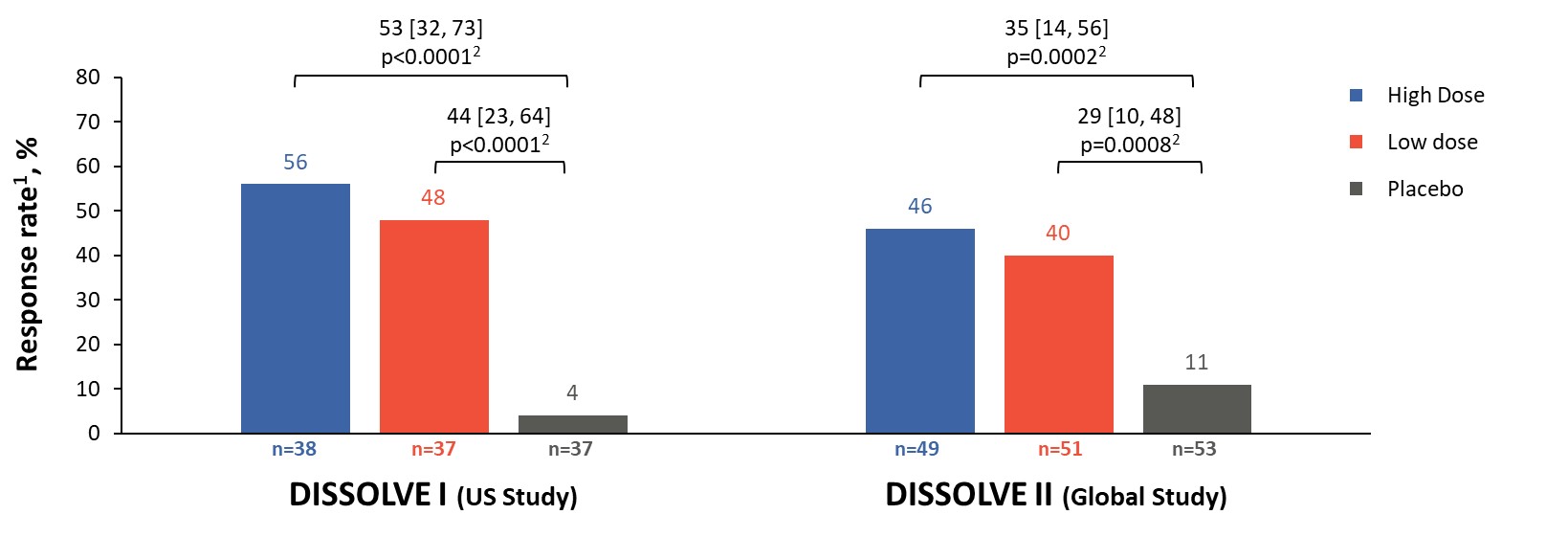
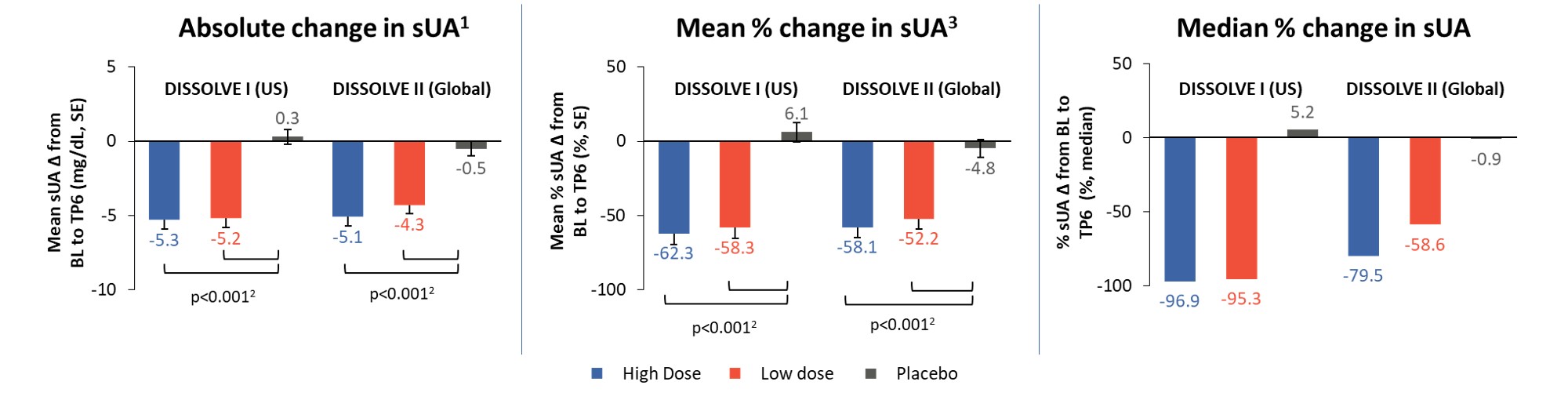
Results: 112 participants (96% male, 66% ≥ 50 years) were enrolled in D1 and 153 (97% male, 72% ≥ 50 years) in D2. Response rates in all treatment groups were significantly different from placebo (p ≤ 0.0008), with 56% and 46% of participants responding in the high-dose group and 48% and 40% in the low-dose group for D1 and D2, respectively (Figure 2). The response rates in participants aged ≥ 50 years were 65% and 47% in the high-dose groups and 47% and 44% in the low-dose groups for D1 and D2, respectively (p ≤ 0.0026 vs placebo). Across all participants in the treatment groups, sUA levels were significantly reduced from baseline (p< 0.001 vs placebo) at TP6 (Figure 3). The safety profile of SEL-212 was favorable, with 3.4% and 4.5% of participants experiencing infusion reactions in the high and low-dose groups, respectively. Reports of gout flares were comparable between treatment groups and placebo. Six participants (3.4%) in the pooled active treatment groups experienced treatment-related serious AEs (n=4 anaphylaxis, n=2 gout flares).
Conclusion: In the DISSOLVE trials, once-monthly treatment with SEL-212 demonstrated statistically significant response rates and reductions in sUA versus placebo. The safety profile of SEL-212 was consistent with that of uricase therapies. Targeted immunomodulation with SEL-212 has the potential to provide a new uricase-based treatment option for patients with gout refractory to conventional therapies.
- Edwards NL. Arthritis Rheum 2008;58(9):2587-90.
- Sands E, et al. Nat Commun 2022;13:272.
To cite this abstract in AMA style:
Baraf H, Kivitz A, Rhodes S, Leung S, Folarin O, Gonzalez-Rivera T, Sobierska J, Christie J, Patel A, DeHaan W, Azeem R, Traber P. Safety & Efficacy of SEL-212 in Patients with Gout Refractory to Conventional Treatment: Primary Outcomes from Two Randomized, Double Blind, Placebo-Controlled, Multicenter Phase 3 Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-efficacy-of-sel-212-in-patients-with-gout-refractory-to-conventional-treatment-primary-outcomes-from-two-randomized-double-blind-placebo-controlled-multicenter-phase-3-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-efficacy-of-sel-212-in-patients-with-gout-refractory-to-conventional-treatment-primary-outcomes-from-two-randomized-double-blind-placebo-controlled-multicenter-phase-3-studies/