Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: Systemic Sclerosis & Related Disorders – Basic Science
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Systemic Sclerosis (SSc) skin fibrosis results in complex changes in transcriptional and signaling pathways in the skin. Through transcription factor activity network analyses, the runt-related transcription factor 1 (RUNX1) has been identified as a key regulator in the skin of individuals with diffuse systemic sclerosis (dSSc). RUNX1 is overexpressed in various human cancers, autoimmune diseases, and other fibrotic conditions; however, the specific contribution of RUNX1 to the pathogenesis of SSc skin fibrosis and the involved signaling pathways remains unknown. We analyzed the specific contributions and function of RUNX1 in SSc.
Methods: RUNX1 expression was analyzed in bulk gene expression data of SSc skin biopsies from multiple cohorts, single-cell RNA sequencing (scRNA-seq) of SSc and control skin, and in sc-multiome data of 3D self-assembled skin equivalent (saSE) tissues. Genome-wide DNA methylation profiles and bulk ATAC-seq data were further generated and analyzed to evaluate the epigenetic state and genome chromatin accessibility of RUNX1 in fibroblasts isolated from dcSSc in 2D and 3D cultures. Fibroblasts in 2D and 3D skin-like tissues were treated with a selective RUNX1 inhibitor, Ro5-3335 or siRNA against RUNX1 followed by collagen contraction, proliferation assays and qRT-PCR for select target genes.
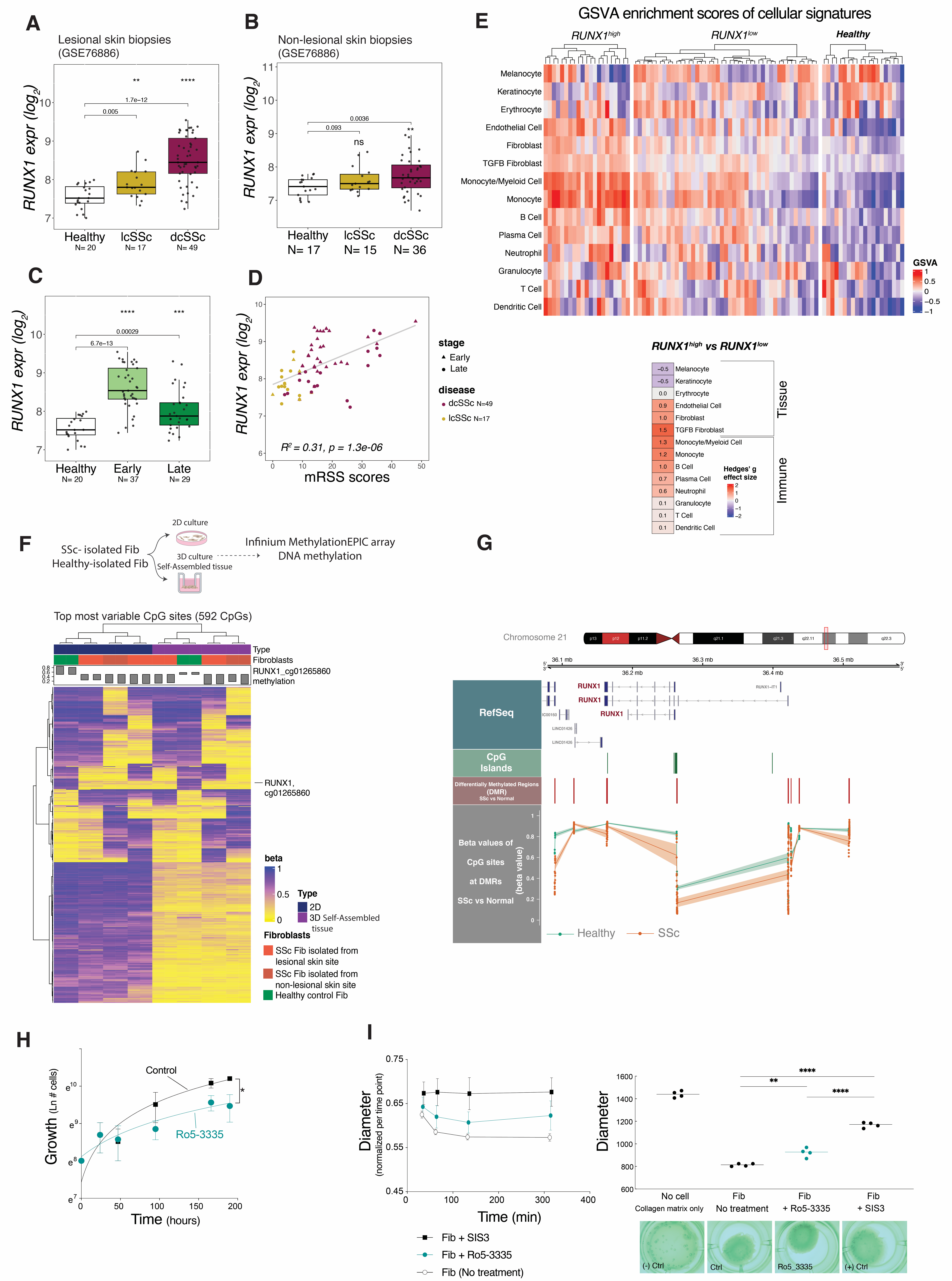
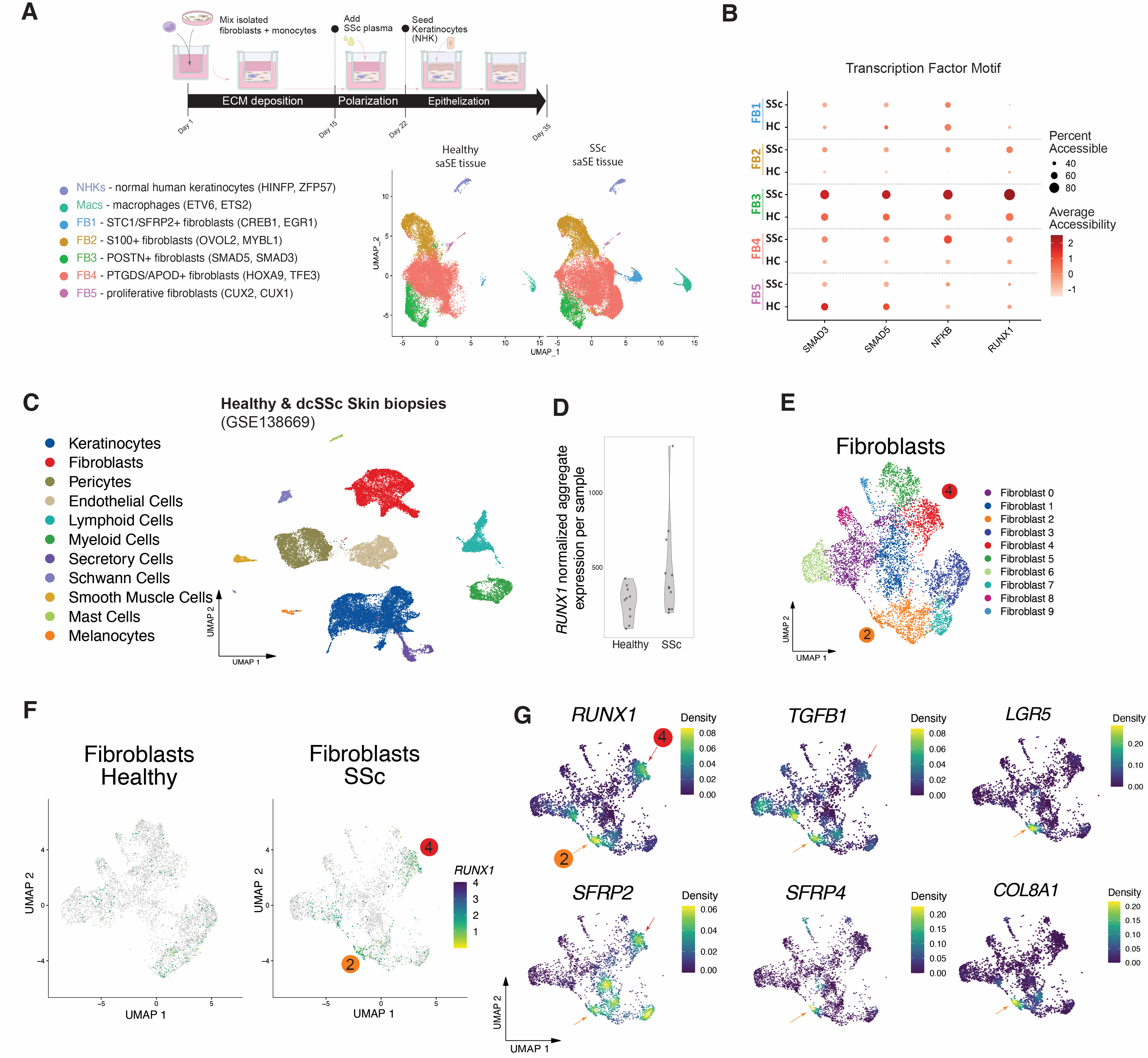
Results: Analysis of gene expression data from multiple cohorts revealed significant over-expression of RUNX1 in skin biopsies of individuals diagnosed with SSc. RUNX1 is also highly expressed in non-lesional skin of patients with SSc (Fig 1A,B). RUNX1 is highly expressed in early disease (Fig 1C), expression is correlated with modified Rodnan Skin Score (mRSS) (r2 = 0.31, p-value = 1.3e-06) (Fig 1D), and it is highly expressed in patients with interstitial lung disease (ILD). The gene set variation analysis (GSVA) scores of TGF-β-activated fibroblast gene signature had the strongest enrichment in RUNX1high SSc patients (Fig 1E). DNA methylation profiling shows RUNX1 hypomethylation in SSc fibroblasts relative to controls (Fig 1F,G). Bulk ATAC-seq of dcSSc fibroblasts showed increased accessibility of RUNX1 chromatin binding. Inhibition of RUNX1 activity by Ro5-3335 significantly reduced the ability of dermal fibroblasts to contract collagen gel matrices and reduced fibroblast proliferation rate (Fig 1H,I). RUNX1 knockdown by siRNA resulted in changes in aSMA, fibronectin (FN1) and COL1A1 expression. Analysis of scRNA-seq and scATAC-seq of in vitro saSE tissues showed increased expression and RUNX1 binding site accessibility in pro-fibrotic POSTN+ fibroblasts (Fig 2A,B). Analysis of scRNA-seq data from SSc and control skin showed enrichment of RUNX1 in profibrotic SSc-enriched fibroblast subpopulations expressing TGFB1, SFRP2, SFRP4, LGR5, LUM, POSTN, COMP, and COL8A1 (Fig 2C-G).
Conclusion: Our findings suggest a link between the extent of dermal fibrosis and the levels of expression of RUNX1. The identification of hypomethylated CpG sites in proximity to the RUNX1 gene implies their potential role in increasing the expression of RUNX1 in fibroblasts. These observations suggest that RUNX1 plays a crucial role in regulating fibroblasts specific to SSc.
To cite this abstract in AMA style:
Parvizi R, Gong Z, Jarnagin H, Abel T, Popovich D, Morrisson M, Wood T, Shenk S, Hinchcliff M, Garlick j, Pioli P, Whitfield M. RUNX1 Is Expressed in a Subpopulation of Dermal Fibroblasts and Is Increased with Systemic Sclerosis Disease Severity [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/runx1-is-expressed-in-a-subpopulation-of-dermal-fibroblasts-and-is-increased-with-systemic-sclerosis-disease-severity/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/runx1-is-expressed-in-a-subpopulation-of-dermal-fibroblasts-and-is-increased-with-systemic-sclerosis-disease-severity/