Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: To estimate the frequency of rheumatic toxicities of immune checkpoint inhibitors (ICI) presenting as de novo or exacerbations of pre-existing rheumatic disease in patients with advanced melanoma. To examine the effect of pre-existing autoimmune and non-immune mediated rheumatic disease on clinical phenotype, treatment course and outcomes and to identify potential risk factors.
Methods: A single-centre observational study of patients with stage III or IV melanoma receiving all available ICI therapies, who completed a for-purpose questionnaire to capture rheumatic symptoms and risk factors upon recruitment and at 12-months. The prevalence of de novo or worsening rheumatic disease was estimated at these 2 time points according to the trajectory of rheumatic symptoms indicated by serial visual analogue scale (VAS) scores for pain and character of rheumatic symptoms. Clinical details were extracted from patients’ medical records. Chi squared tests of heterogeneity, univariate and multivariate analysis were employed to identify associations with pre-morbid, melanoma- and treatment-related factors.
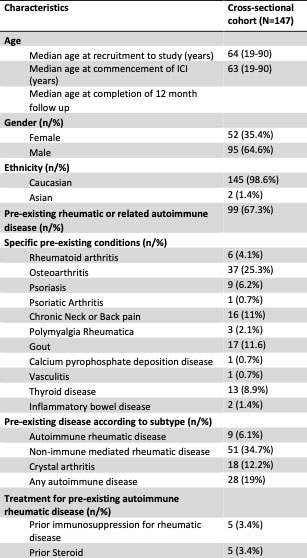
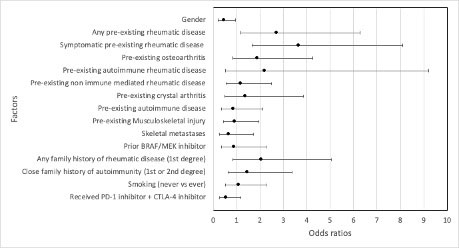
Results: Amongst 147 eligible patients, pre-existing rheumatic or related autoimmune diseases was reported in 67.3% of patients; osteoarthritis (25.3%) was the most common, followed by chronic axial pain (11%), and 9 patients (6.1%) had pre-existing autoimmune rheumatic conditions (Table 1). At the time of recruitment, the median time since commencing ICIs was 9 months (range 0-64 months). The prevalence of new or worsening rheumatic symptoms such as peripheral joint, axial or muscle pain or muscle weakness was 32.4%, including 7 patients (5.0%) with de novo rheumatic symptoms and 38 patients (27.3%) with exacerbation of pre-existing rheumatic symptoms. The incidences of clinician-documented rheumatic syndromes such as arthralgia, inflammatory arthritis, PMR-like syndrome and inflammatory myopathy were 39.5%, 5.4%, 3.4% and 0.7% respectively. Amongst 37 patients with pre-existing osteoarthritis, 56.8% developed worsening arthralgia, 8.1% developed inflammatory arthritis and 5.4% developed PMR-like syndrome. Two thirds of patients with pre-existing autoimmune rheumatic disease developed either disease exacerbation or a new rheumatic disease. Pre-existing rheumatic disease conferred a 2.7-fold increase in odds of developing new or worsening rheumatic symptoms (OR 2.714, 95% CI 1.173 – 6.278), whilst the presence of symptomatic pre-existing rheumatic disease conferred a 3.6-fold increase in odds (OR 3.66, 95% CI 1.661 – 8.092). Binary logistic regression identified pre-existing symptomatic rheumatic disease, as the primary risk factor for developing rheumatic toxicities (OR 3.161 95% CI 1.362 – 7.338, p = 0.007).
Conclusion: De novo or worsening rheumatic disease symptoms occur commonly amongst patients with advanced melanoma treated with ICI therapy and includes exacerbations of both autoimmune and non-immune mediated rheumatic conditions. Patients with pre-existing rheumatic conditions are 3.6 times more likely to develop exacerbations or new rheumatic disease features if they are symptomatic prior to commencing ICI therapy.
To cite this abstract in AMA style:
Bruce A, Long G, Menzies A, Fernandes B, Joshua F. Rheumatic Toxicities of Immune Checkpoint Inhibitors in Patients with Advanced Melanoma and the Effect of Pre-existing Autoimmune and Non-immune Mediated Rheumatic Conditions [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/rheumatic-toxicities-of-immune-checkpoint-inhibitors-in-patients-with-advanced-melanoma-and-the-effect-of-pre-existing-autoimmune-and-non-immune-mediated-rheumatic-conditions/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatic-toxicities-of-immune-checkpoint-inhibitors-in-patients-with-advanced-melanoma-and-the-effect-of-pre-existing-autoimmune-and-non-immune-mediated-rheumatic-conditions/