Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Limited cutaneous systemic sclerosis (lcSSc) is the most frequent subset of scleroderma, yet there is a paucity of outcome measures available to assess symptoms important to patients with lcSSc. The CRISTAL project is an international initiative that aims to create a combined response index for lcSSc for use in clinical trials: the CRISTAL index. The most bothersome and/or common domains in lcSSc were identified through focus groups, and existing outcomes were identified through a scoping literature review. The next steps of this CRISTAL initiative were a) to select the most appropriate existing clinician-reported outcomes (ClinROs) and performance outcomes (PerfOs) for the assessment of lcSSc; and b) to create a disease-specific patient-reported outcome measure (PROM) assessing the most common and/or bothersome lcSSc symptoms.
Methods: Experts were asked to suggest additional items/outcome measures beyond those identified through the scoping review in a two-round international online Delphi exercise. ClinROs and PerfOs were then selected during a two-day meeting utilizing a nominal group technique (NGT) comprising 3 patient research partners and 8 international experts. The CRISTAL PROM was created using a patient-centered approach, with item elicitation based on the focus groups, item selection through a patient-centered online survey, and preliminary validation through cognitive debriefings with patients with lcSSc.
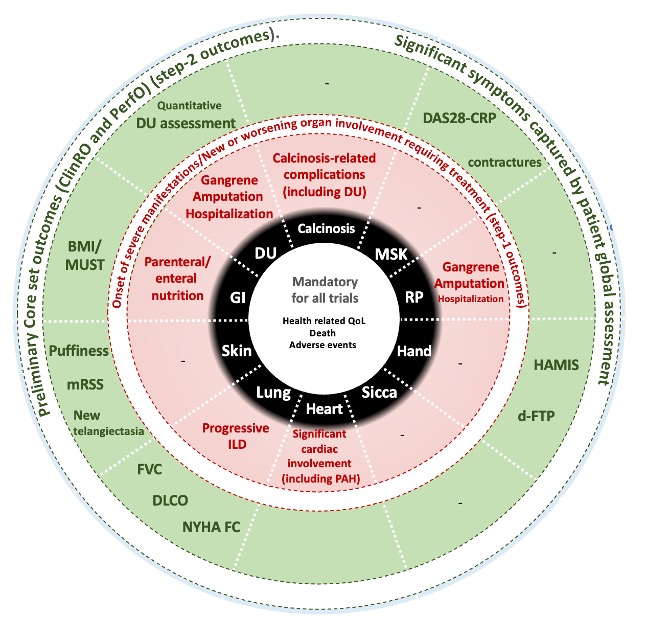
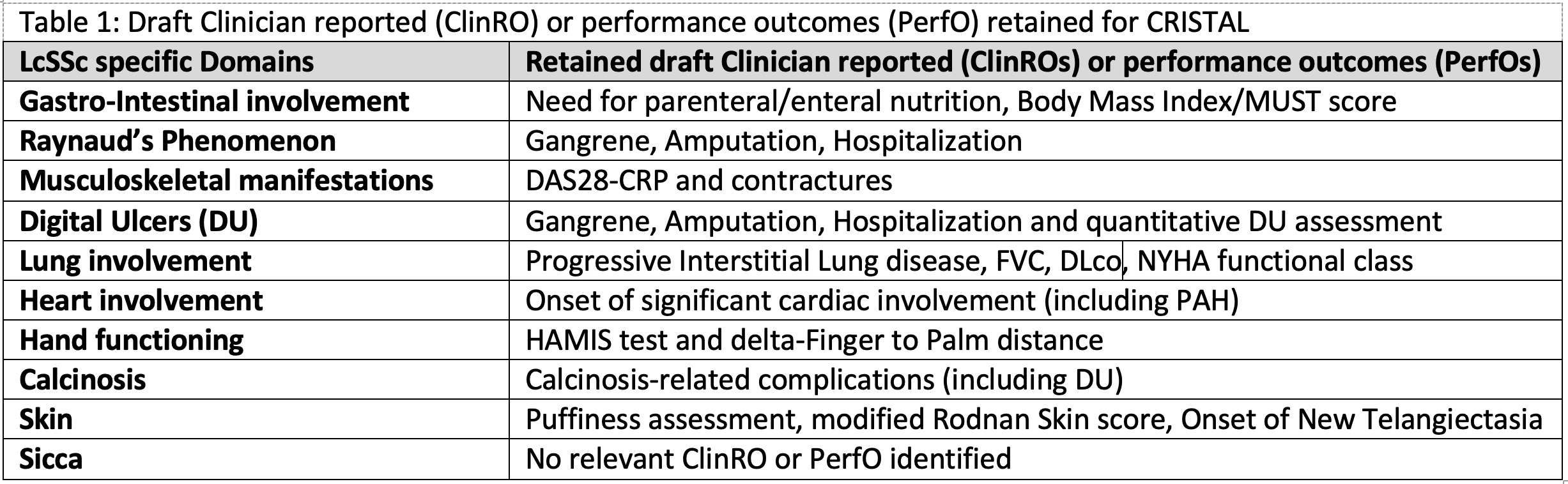
Results: 100 experts were invited to participate in the Delphi exercise and 71 provided answers to round 1. Participants from round 1 were invited to round 2, rating each item on scales (range: 1-9) for feasibility, face validity, content validity, and overall appropriateness for the CRISTAL Index. 59/71 participants provided answers to round 2. Items endorsed by > 50% of the experts were included for discussion at the 2-day NGT discussion. During the NGT meeting voting members discussed and ranked items from “most appropriate for the CRISTAL index” to “least appropriate”. After each ranking, voting members were asked if they agreed with the ranking; 80% agreement was required to move to the next domain. Across the 10 lcSSc-specific domains discussed, 19 items (17 ClinROs and 2 PerfOs) were identified and retained as draft items (Figure 1).
In parallel, the preliminary CRISTAL PROM was created and included 37 items assessing symptoms experience from 4 general and 8 lcSSc specific domains with a recall period of 7 days.
Conclusion: The international CRISTAL initiative used a patient-centered approach to select the most relevant ClinROs and PerfOs assessing common and/or bothersome domains in patients with lcSSc. In parallel, a new CRISTAL PROM was created. The next steps of the CRISTAL initiative will include assessment of the psychometric properties of the selected ClinRO & PerfOs, and the preliminary CRISTAL PROM in longitudinal cohorts to develop the final CRISTAL index.
Acknowledgments: CRISTAL is partly supported by the grants from World Scleroderma Foundation, Scleroderma & Raynaud’s UK, the Scleroderma Clinical Trial Consortium, and the Scleroderma Research Foundation.
To cite this abstract in AMA style:
Lescoat A, Chen Y, Murphy S, Vann N, Kortam N, Gedert R, Farrington S, Allanore Y, Cella D, Chung L, Clements P, Denton C, Del Galdo F, Distler O, Hinchcliff M, Hughes M, Hummers L, Pauling J, Pope J, Steen V, Varga J, Merkel P, Buch M, Khanna D. Results from Outcomes Selection and Development of a Patient-reported Outcome Measure for a Combined Response Index for Limited Cutaneous SSc: The CRISTAL Project [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/results-from-outcomes-selection-and-development-of-a-patient-reported-outcome-measure-for-a-combined-response-index-for-limited-cutaneous-ssc-the-cristal-project/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/results-from-outcomes-selection-and-development-of-a-patient-reported-outcome-measure-for-a-combined-response-index-for-limited-cutaneous-ssc-the-cristal-project/