Session Information
Date: Tuesday, October 28, 2025
Title: (1990–2014) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients (pts) with uncontrolled gout (UG) and tophi experience joint pain, impaired function and poor quality of life (Schlesinger et al. Semin Arthritis Rheum 2020; Dalbeth et al. Nat Rev Dis Primers 2019; Dalbeth et al. Lancet 2021). With oral urate-lowering therapies, tophus resolution may take several years despite optimal dosing (Laurent et al. RMD Open 2023). NASP (formerly SEL-212) is a novel, every 4-week, sequential infusion therapy consisting of targeted immunomodulating, nanoencapsulated sirolimus (NAS; formerly SEL-110) co-administered with a pegylated uricase (formerly SEL-037) designed to reduce serum uric acid (sUA) levels in pts with UG. Here, we report pooled tophus outcomes from the DISSOLVE studies.
Methods: This post hoc analysis pooled data from DISSOLVE I II ((NCT04513366, NCT04596540) in the intent-to-treat (ITT) population and in pts who received 6 doses of NASP (high-dose [HD]: sequential infusions of 0.15 mg/kg NAS and 0.2 mg/kg pegadricase; low-dose [LD]: sequential infusions of 0.10 mg/kg NAS and 0.2 mg/kg pegadricase) or placebo (PBO). Tophi response was a predefined secondary endpoint. Percent tophus response included partial response (PR), defined as ≥50% and < 100% reduction in the area of a tophus without enlargement of any existing tophus and no new tophus, and complete response (CR), defined as 100% reduction in area of or complete disappearance of a tophus without enlargement of any existing tophus and no new tophus. A triple read model of tophus evaluation was established after unblinding to improve the quality of measuring tophus size and improve data handling in instances of images with quality issues.
Results: In the ITT population (among pts with tophi at baseline (BL): n=55 [HD]; n=55 [LD]; n=57 [PBO]), tophus response of PR or better was observed in 82% in HD (p=0.0077), 88% in LD (p=0.0016), vs 51% in PBO by week 24. NASP treatment resulted in a clinically relevant response of CR in significantly more patients receiving NASP compared with PBO by week 24: HD, 31% (p=0.0044); LD, 48% (p=0.0001), vs PBO, 5%. In the subset of pts with tophi at BL who received 6 doses of treatment (n=23 [HD]; n=22 [LD]; n=42 [PBO]), a significantly greater proportion of patients on NASP experienced CR by week 24: HD, 49% (p=0.0002); LD, 70% (p< 0.0001), vs PBO, 5%. Consistent with the correlation between tophus reduction and sUA reduction, patients who received 6 doses of HD NASP had 89% lower sUA than PBO-treated patients at Day 168 (end of study) with a similar response for LD NASP (Figure 1).
Conclusion: Treatment with NASP resulted in a greater tophus response compared to PBO. In addition, a robust sUA reduction, a key factor in tophus resolution, was observed in NASP-treated pts. These results highlight the effectiveness of NASP in lowering sUA and promoting tophus resolution.
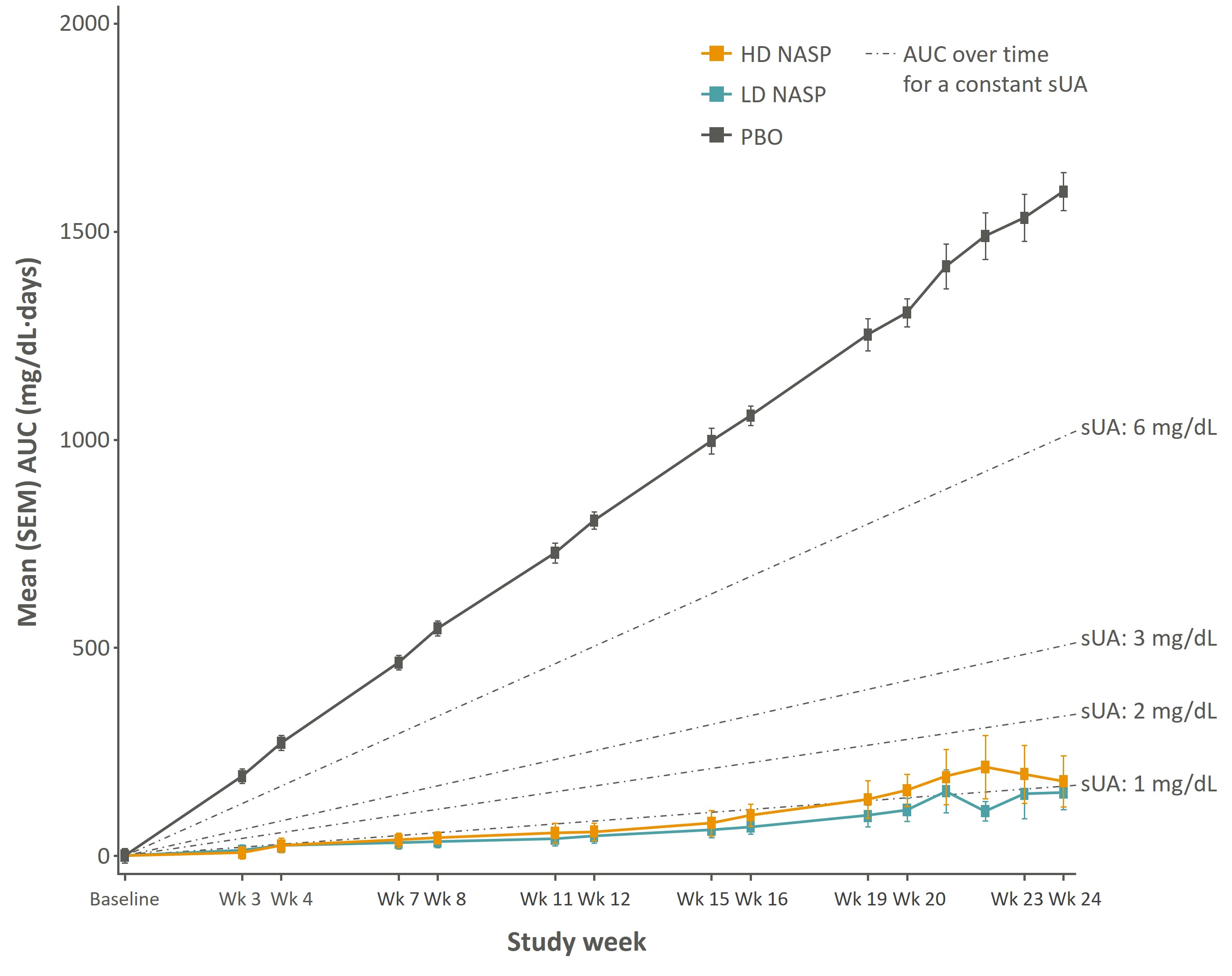 Figure 1. Mean cumulative sUA AUC through week 24 in patients who completed 6 doses of NASP or PBO with tophi at baseline.
Figure 1. Mean cumulative sUA AUC through week 24 in patients who completed 6 doses of NASP or PBO with tophi at baseline.
Cumulative sUA is a measure of the duration and intensity of sUA exposure over time, defined in this study as AUC at each time point (average of the current and previous sUA values multiplied by the time between the current and previous sUA values). The cumulative sUA AUC at each study week in the figure is the total AUC from day 1 up to that week. SUA assessments were performed at the end of each week. Mean (±SEM) is shown for pts with tophi at baseline who completed 6 doses of HD NASP (yellow), LD NASP (teal) or placebo (dark gray). Dashed lines show what the cumulative area under the curve would be at each point in time for a subject that had constant sUA level.
AUC, area under the curve; HD NASP, sequential infusions of 0.15 mg/kg nanoencapsulated sirolimus and 0.2 mg/kg pegadricase, LD NASP, sequential infusions of 0.10 mg/kg nanoencapsulated sirolimus and 0.2 mg/kg pegadricase; PBO, placebo; SEM, standard error of mean; sUA, serum uric acid; Wk, week.
To cite this abstract in AMA style:
Baraf H, Khanna P, Lioté F, Azeem R, DeHaan W, Peace B, Santin-Janin H, Desai B, Kivitz A. Reduction in Tophi Observed in Patients with Uncontrolled Gout Treated with NASP: Results from Phase 3 DISSOLVE Studies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/reduction-in-tophi-observed-in-patients-with-uncontrolled-gout-treated-with-nasp-results-from-phase-3-dissolve-studies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduction-in-tophi-observed-in-patients-with-uncontrolled-gout-treated-with-nasp-results-from-phase-3-dissolve-studies/
