Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The Toll-Like Receptor family (except TLR3) signal through IRAK4 and IRAK1 to produce an array of cytokines (including IL-6, IL-23 and TNFα in response to pathogen and damage associated molecular patterns (PAMPs and DAMPs).The IRAK proteins are also involved in the signaling cascade of the IL-1 receptor family; therefore, they play a critical role in innate immune response controlling chronic inflammation. As a result, inhibition of IRAK4 has been investigated as a means of attenuating a range of autoimmune diseases including rheumatoid arthritis, with zimlovisertib demonstrating encouraging results in a Phase 2 rheumatoid arthritis study. We identified R835, which has shown proof of mechanism in an LPS challenge study in healthy human volunteers. In this study we report an improved molecule R851 which has been modeled to require five-fold lower dosing for similar efficacy making it suitable for chronic indications such as rheumatoid arthritis.
Methods: Prioritizing inhibition of TLR signaling in a cell-based assay in conjunction with IRAK4 biochemical inhibition, we identified a series of potent and selective dual IRAK1 and IRAK4 inhibitors. The results suggested that dual IRAK1/4 inhibition results in a more effective inhibition of TLR signaling than IRAK4 alone. Having identified R835, we undertook a retrospective analysis of our IRAK1/4 inhibitor portfolio to identify R851.
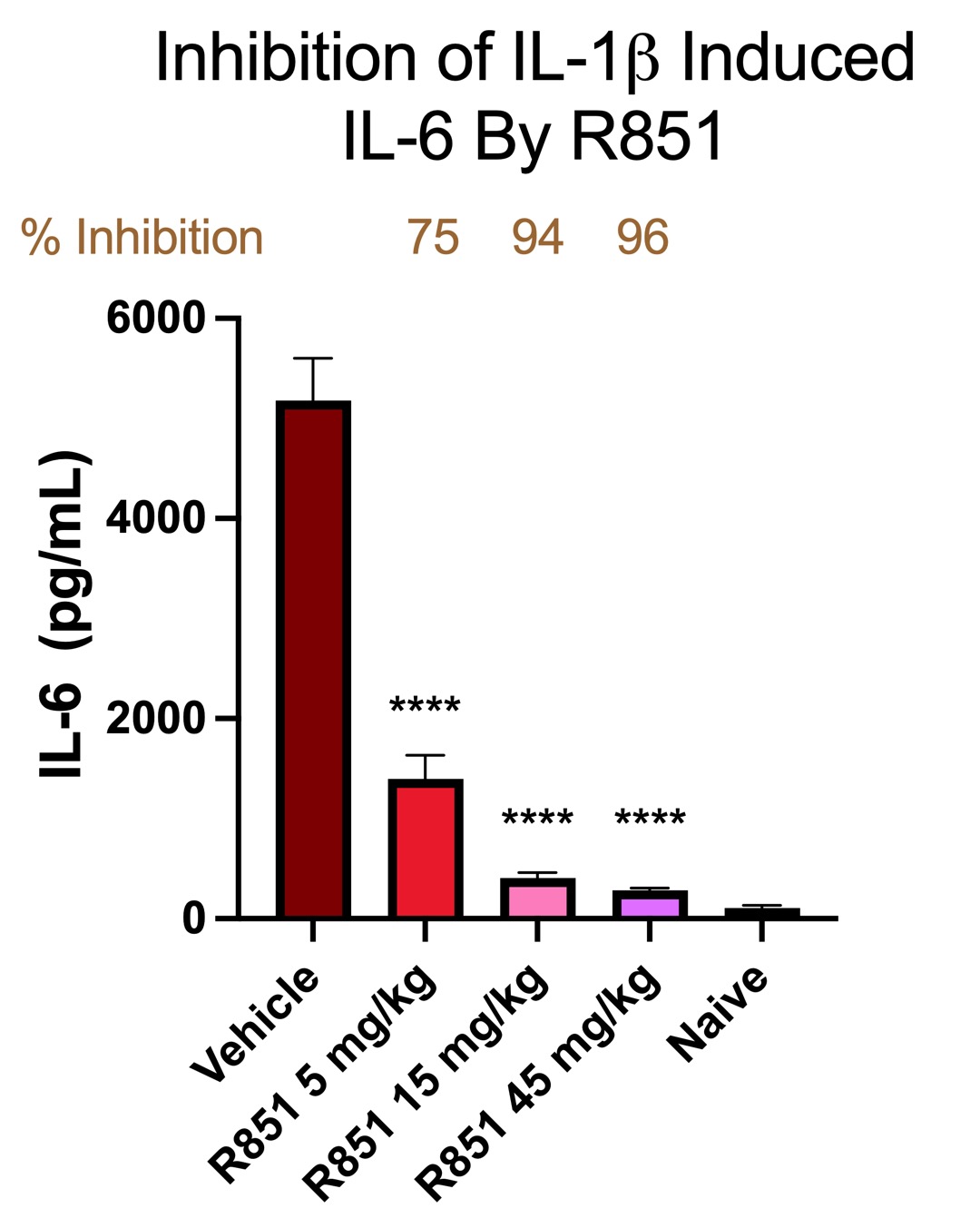
Results: Our first molecule R835, currently being developed for low-risk myelodysplastic syndrome, demonstrated robust efficacy in a number of animal models including a collagen induced arthritis model.R835 not only inhibited disease when dosed prophylactically but also reversed symptoms when dosing was initiated after onset of disease. In the clinic, R835 showed dose-dependent, linear PK and proof of mechanism in humans. While R835 is a promising IRAK1/4 inhibitor with clinical potential, we hoped to identify an alternative molecule with favorable pharmacokinetic characteristics and increased potency for chronic autoimmune indications such as rheumatoid arthritis. A retrospective analysis of the data from the IRAK1/4 inhibitors generated over the program led to R851, a molecule from the R835 scaffold that showed improved potency in both cellular and biochemical assays. Further, R851 demonstrated 7-10 fold improvement in potency in our whole blood assays, driven by reduced plasma protein binding. Building on our understanding of the scaffold we expect R851 to require 5-fold less exposure for a similar effect to R835.The compound has been taken through PK studies across species demonstrating a dose-dependent, linear and predictable PK profile in multiple day dosing. In vivo, R851 shows a robust inhibition of IL-6 after IL-1β stimulation (Fig. A) which is expected to translate into efficacy in chronic models.
Conclusion: Using the knowledge gained in the development of R835, we have identified R851 as a second generation dual IRAK1 and IRAK4 inhibitor. The increased potency in whole blood assays is believed to be driven by a reduction in protein binding. We predict that R851 will require a significantly lower exposures for efficacy in humans which should position this molecule for a chronic condition like rheumatoid arthritis.
To cite this abstract in AMA style:
Chen Y, Yi S, Markovtsov V, Samant B, Chow A, Masuda E, Shaw S. R851, a Potent Second Generation IRAK1 and IRAK4 Inhibitor Suppresses IL-6 in Vitro and in Vivo for the Treatment of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/r851-a-potent-second-generation-irak1-and-irak4-inhibitor-suppresses-il-6-in-vitro-and-in-vivo-for-the-treatment-of-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/r851-a-potent-second-generation-irak1-and-irak4-inhibitor-suppresses-il-6-in-vitro-and-in-vivo-for-the-treatment-of-rheumatoid-arthritis/