Session Information
Date: Sunday, November 13, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster II: Manifestations
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Stimulation of the kynurenine/tryptophan pathway by interferons alpha and gamma, both known contributors to SLE pathogenesis, leads to a potentially neurotoxic imbalance of pathway metabolites quinolinic acid (QA), a NMDA receptor agonist, and kynurenic acid (KA), a NMDA receptor antagonist. Elevated QA/KA ratios are implicated in pathogenesis of cognitive dysfunction (CD) in neurodegenerative diseases [1], including SLE.[2] Using FDG-PET and diffusion tensor MRI (DTI) we previously demonstrated brain regions with abnormal increased or decreased metabolism and clusters of decreased white matter structural integrity (WMI), respectively, in SLE compared to healthy controls (HC).[3] Imaging data from a subset of these SLE and HC were analyzed with serum QA/KA ratios and cognitive testing to determine associations between QA/KA ratios and brain structure and function.
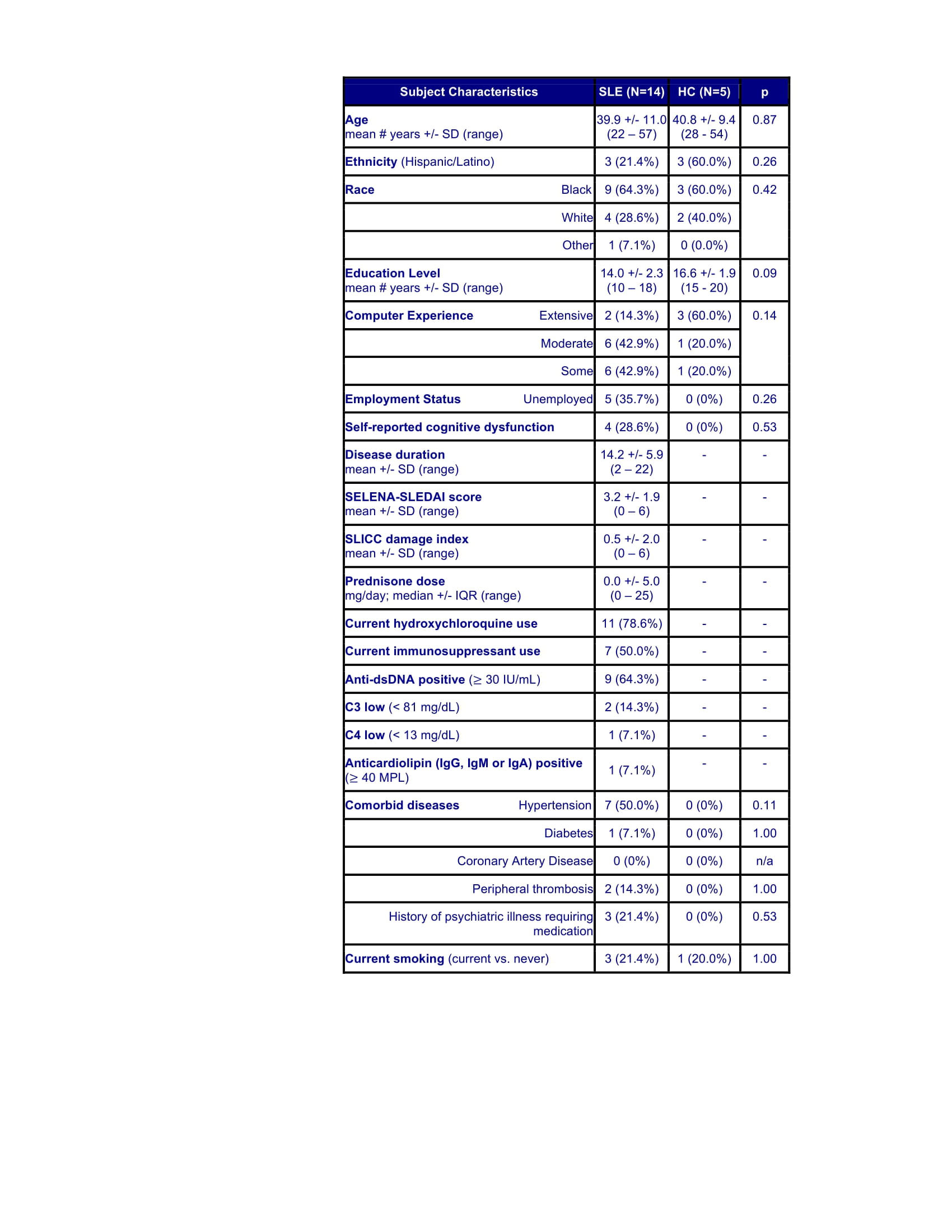
Methods: Brain imaging data from 14 SLE and 5 HC subjects were analyzed with serum metabolite levels (measured with high-performance liquid chromatography with mass spectrometry) and cognitive testing (assessed with the Automated Neuropsychological Assessment Metrics) obtained within 2 years of imaging except 2 SLE where the interval was 4 years. SLE subjects met ACR criteria, had low disease activity and corticosteroid doses (Table) and no history of CNS insult. Individual mean fractional anisotropy (FA) values for 6 previously identified abnormal WM clusters and mean metabolic values for previously identified abnormal regions were measured, and WM pathways were constructed between regions with reduced FA, as previously described [3]. Differences between SLE and HC were assessed using t-tests. Correlations between QA/KA and metabolism and FA were assessed with Pearson’s or Spearman’s correlations, as appropriate. Regression analysis assessed relationships among metabolism, FA, cognitive performance and QA/KA.
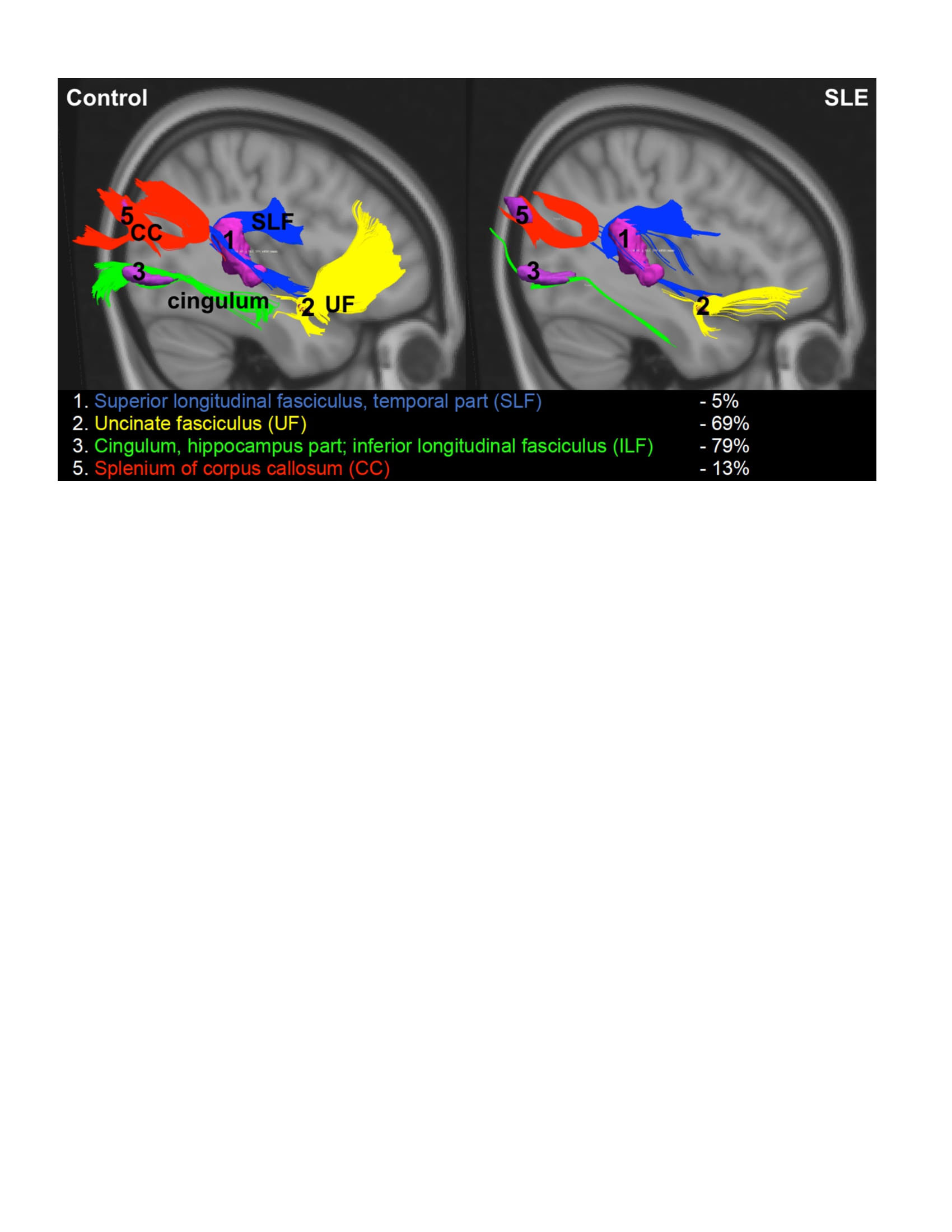
Results: Clusters of reduced FA in SLE vs. HC were found in the superior longitudinal fasciculus (SLF, 0.32 +/- 0.03 vs. 0.36 +/- 0.05, p=0.046), uncinate fasciculus (0.23 +/- 0.04 vs. 0.32 +/- 0.06, p=0.001) and SLF and corpus callosum combined (SLF/CC, 0.32 +/- 0.05 vs. 0.37 +/- 0.05, p=0.039), and WM tracts between clusters were diminished in SLE (Figure). Elevated QA/KA correlated with decreased FA in the CC (r = -0.58, p=0.040), the SLF/CC (r = -0.64, p=0.018) and with increased Simple Reaction Time (SRT, r = 0.72, p=0.006). The combination of elevated QA/KA and decreased FA in SLF/CC predicted worse SRT (R2=0.48, p=0.009). Elevated QA/KA correlated with decreased left premotor cortex (PMC) metabolism (r = -0.61, p=0.021) and increased SRT (r = 0.74, p=0.003). The combination of elevated QA/KA and decreased left PMC metabolism predicted worse SRT (R2=0.73, p< 0.001).
Conclusion: Elevated QA/KA ratios correlated with decreased PMC metabolism and WMI in fronto-parietal pathways, suggesting neuronal and associated WM damage. The combination of elevated QA/KA and abnormalities in these brain regions correlated with increased SRT, a measure of visuomotor processing speed and attention. These results, which require validation in a larger longitudinal cohort, suggest a role for QA, and associated neurotoxicity, in CD in SLE.
References:
1. Schwarcz R and Stone TW. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017;112:237_247.
2. Anderson EW, Fishbein J, Hong J, et al. Quinolinic acid, a kynurenine/tryptophan pathway metabolite, associates with impaired cognitive test performance in systemic lupus erythematosus. Lupus Sci Med 2021;8.
3. Mackay M, Vo A, Tang CC, et al. Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment. JCI Insight 2019;4.
To cite this abstract in AMA style:
Anderson E, Tang C, Vo A, Aranow C, Volpe B, Diamond B, Mackay M. Quinolinic Acid, a Kynurenine/Tryptophan Pathway Metabolite, Correlates with Abnormalities in Brain Structure and Function in SLE [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/quinolinic-acid-a-kynurenine-tryptophan-pathway-metabolite-correlates-with-abnormalities-in-brain-structure-and-function-in-sle/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quinolinic-acid-a-kynurenine-tryptophan-pathway-metabolite-correlates-with-abnormalities-in-brain-structure-and-function-in-sle/