Session Information
Date: Monday, November 13, 2023
Title: (0859–0885) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Osteoarthritis (OA) has long been thought to be only of local mechanical origin. However, it has been shown recently that there is a phenotype of OA with inflammatory properties. Here, we hypothesize that circulating neutrophils could be involved in disease pathogenesis suggesting a systemic involvement in this “local” disease.
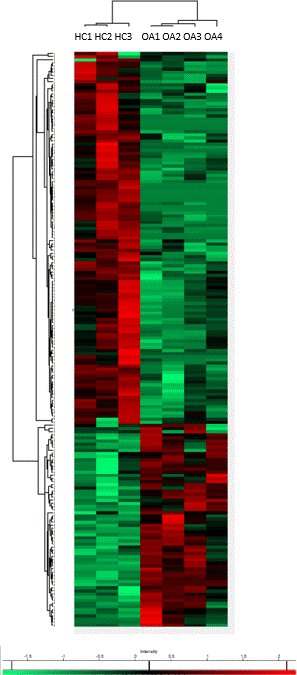
Methods: We investigated the cytosolic proteome of blood neutrophils from four OA patients with an inflammatory knee osteoarthritis and compared it to the cytosolic proteome of blood neutrophils from three healthy controls (HC) of similar age. Functional analysis was generated through the use of the software Ingenuity Pathway Analysis (IPA). We further investigated in vitro the physiological apoptosis of isolated circulating neutrophils from OA patients compared to neutrophils from HC and measured the glycogen content in neutrophil cytosol.
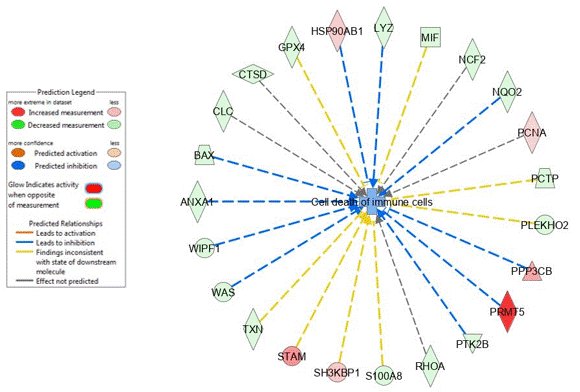
Results: Based on the differentially regulated proteins, IPA predicts a downregulation of the glycogen degradation pathway as the most important difference between blood neutrophils from OA patients and those from HC, suggesting a disturbance in glycogen storage or use in OA neutrophils. Further, IPA predicts an inhibition of cell death of immune cells and an activation of senescence of cells. One of the differentially regulated proteins involved in survival is PCNA, which is found to be increased in neutrophils from OA patients. When measuring the glycogen content in blood neutrophils from OA patients compared with that from HC, we found no difference in glycogen content. However, we found a significant difference in the content of glycogen between neutrophils at a basal state and apoptotic neutrophils in both groups, suggesting that neutrophils use glycogen during their apoptosis. There was no correlation between the basal glycogen concentration of the cells and the percentage of apoptosis in neutrophils from both HC and OA, suggesting that the initial glycogen concentration has not a direct impact on the process of apoptosis of neutrophils. Preliminary results of Annexin-V labeling showed that neutrophils from OA patients have a significant delayed apoptosis compared with HC.
Conclusion: Our findings uncover for the first time a dysregulation in circulating neutrophils in OA that could have an impact on the disease pathogenesis and demonstrate a systemic involvement in OA. We provide evidence that cytosolic proteins of circulating neutrophils of OA patients are disturbed in terms of glycogen metabolism and apoptosis, which might result in a higher survival rate. Molecular mechanisms underlying these disturbances remain to be elucidated.
To cite this abstract in AMA style:
Amsler J, Le Gall M, Daste C, Rannou F, Witko-Sarsat V. Proteomic Analysis of Circulating Neutrophils from Osteoarthritis Patients Shows Metabolic Dysregulation That Is Associated with Decreased Physiologic Apoptosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/proteomic-analysis-of-circulating-neutrophils-from-osteoarthritis-patients-shows-metabolic-dysregulation-that-is-associated-with-decreased-physiologic-apoptosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/proteomic-analysis-of-circulating-neutrophils-from-osteoarthritis-patients-shows-metabolic-dysregulation-that-is-associated-with-decreased-physiologic-apoptosis/