Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: A definition of primary heart involvement (pHI) in systemic sclerosis (SSc) was recently developed (Bruni C et al. J Scleroderma Relat Disord. 2022;7:24-32). Cardiac magnetic resonance (CMR) imaging was selected as the non-invasive diagnostic modality of choice to characterize myocardial involvement in SSc. In this study, we aimed to evaluate the prognostic value of CMR-supported diagnosis of SSc-pHI in a real-life SSc-cohort.
Methods: We included patients from our SSc cohort fulfilling the ACR/EULAR classification criteria for SSc, who underwent at least one CMR and had follow-up data. Study baseline was defined as the date of the CMR, or the date of the first pathologic CMR in patients with multiple exams. Based on CMR and additional cardiologic tests, patients were divided into four groups: no heart disease, SSc-pHI, other heart disease, and combined SSc-pHI and other heart disease.
Our main, combined outcome included death or cardiac events (any of: hospitalization due to heart failure, new arrhythmias, new diastolic dysfunction, new left ventricular ejection fraction < 50%, new dyspnea NYHA class III or IV) during follow up. To evaluate the risk posed by SSc-pHI, we performed a Kaplan-Meier survival analysis for the main combined outcome, as well as for death and for cardiac events separately. This was followed by multivariable Cox regression models, including the cardiac disease groups, and other risk factors for adverse prognosis in SSc.
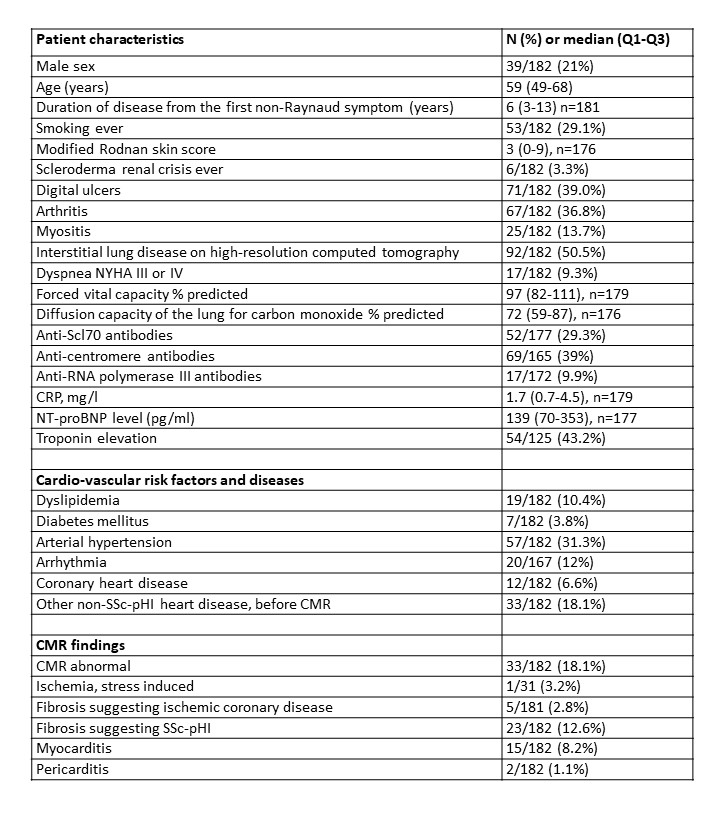
Results: Among 182 patients included, 39 (21%) were males, 54 (30%) had diffuse cutaneous SSc, 6 (3%) had history of scleroderma renal crisis (SRC), 24 (13%) had pulmonary hypertension (PH) by right heart catheterization, 92 (50%) had interstitial lung disease (ILD), of whom 33% significant, defined as 20% extent on high-resolution computer tomography or forced vital capacity < 70% predicted, and 33 (18%) had non-SSc related cardiac conditions already diagnosed before CMR (Table 1). Based on cardiac assessments including CMR, patients were classified as no heart disease (88, 48%), SSc-pHI (23, 13%), other heart disease (60, 33%), and both SSc-pHI and other heart disease (11, 6%).
During a median (Q1-Q3) follow-up of 7 (3-9) years, the combined outcome occurred in 79 (43.4%) patients. Specifically, death occurred in 37 (20%) patients. Cardiac events occurred in 56 (30.8%) patients: 14 arrhythmias, 4 LVEF< 50%, 28 diastolic dysfunction, 6 hospitalizations due to heart failure and 20 deterioration of NYHA class. No patients developed sustained ventricular tachycardia/fibrillation.
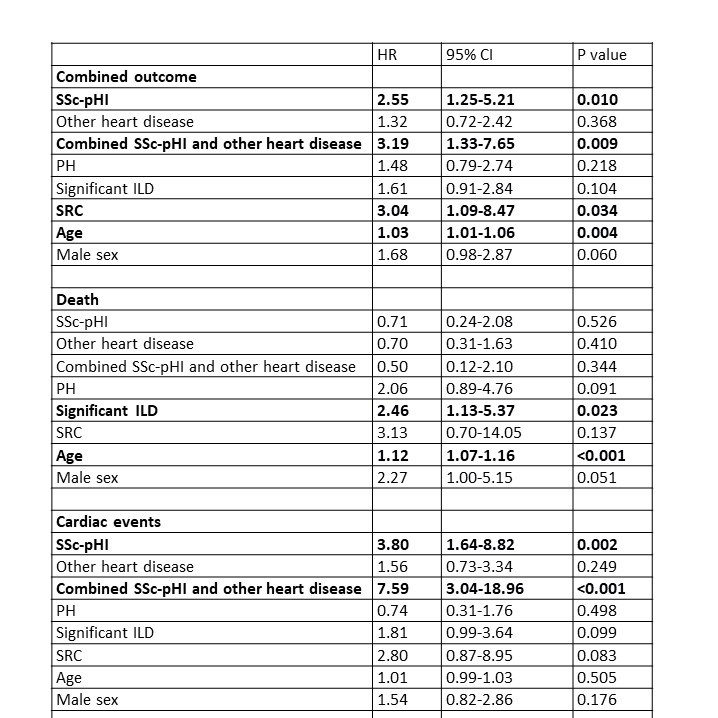
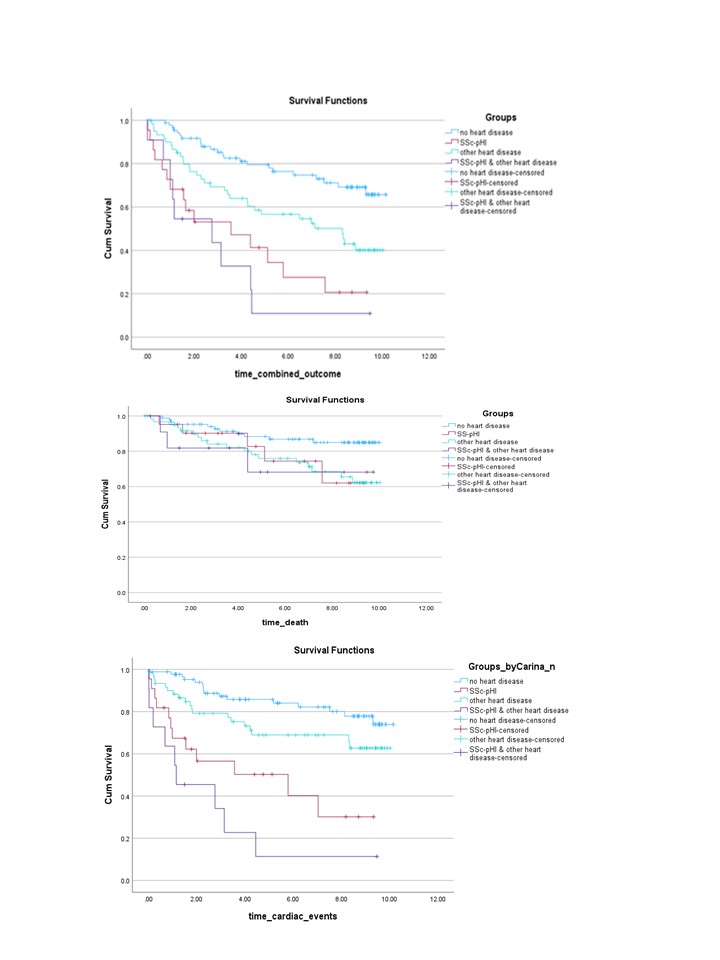
Both in Kaplan-Meier survival curves (Figure 1) and in Cox regression models adjusted for age, sex, significant ILD, PH and history of SRC, SSc-pHI was an independent risk factor for the combined outcome and for cardiac events alone, but not for death (Table 2).
Conclusion: SSc-pHI is associated with severe prognosis and is a risk factor for cardiac outcomes, independently of other concomitant heart conditions and extra-cardiac SSc manifestations. Patients with SSc-pHI should be given special attention, and there is an urgent need for studies on treatment of SSc-pHI.
The Hazard Ratios for the cardiac groups are presented with “no heart disease” as reference.
To cite this abstract in AMA style:
Gharibian C, Lupi V, Gotschy A, Becker M, Dobrota R, Elhai M, Muraru S, Jordan S, Hoffmann-Vold A, Distler O, Manka R, Bruni C, Mihai C. Prognostic Value of Systemic Sclerosis-associated Primary Heart Involvement [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/prognostic-value-of-systemic-sclerosis-associated-primary-heart-involvement/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prognostic-value-of-systemic-sclerosis-associated-primary-heart-involvement/