Session Information
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) have improved cancer therapy [1] by inducing a higher immune system activity and subsequent attack of tumor cells. However, this effect can cause rheumatic immune-related adverse events (rh-irAE), which have not yet been extensively studied.
Methods: We analysed 437 patients between January 2014 and October 2019, treated with ipilimumab (anti-CTLA-4) and / or nivolumab (anti-PD-1) or pembrolizumab (anti-PD-1) at the Department of Oncology, Hematology and Rheumatology at the University Hospital in Bonn, Germany.
Results: Of the 437 patients, 260 (60%) were males, 177 (40%) were females with a mean age of 64 years (SD ± 14) at the beginning of the ICI-therapy. 163 patients (37.3%) displayed at least one irAE, including rh-irAE. Most common other non-rheumatic irAE (non-rh-irAE) were rash, colitis and hepatitis.
We identified 19 patients (4.3%) with a minimum of one rh-irAE due to ICI-therapy, three of those had a pre-existing rheumatological disease. Clinical characteristics of patients with rh-irAE, including types of rh-irAE and therapy, are displayed in table 1. Rh-irAE occurred in one patient (2.6%) with ipilimumab, in ten patients (5.3%) with nivolumab and in eight patients (5.0%) with pembrolizumab. Thirteen patients with rh-irAE received ICI-therapy (percentage within each malignancy given in brackets) for melanoma (6.2%), three patients for lung cancer (3.8%), two patients for head and neck tumor (5.9%) and one patient for gastrointestinal carcinoma (8.3%). Most rh-irAE were classified as moderate severe (CTCAE [Common Terminology Criteria of Adverse Events] grade 2: 68.4%). Even though patients benefited from ICI treatment, therapy had to be discontinued in six of them as a consequence of rh-irAE.
Interestingly, patients with rh-irAE had a significantly higher tumor response rate compared to patients without irAE (94.4% vs. 32.5%; p< 0.0001). The comparison of best tumor response in different cohorts (patients without irAE, with non-rh-irAE and with rh-irAE) is listed in table 2. In order to calculate progression-free survival (PFS), we performed a Kaplan-Meier analysis (Fig. 1) and compared thereby the PFS in the three cohorts mentioned above. Using the log-rank test, a significant difference in PFS was found in these three cohorts (p=0.003).
Conclusion: Rh-irAE occur under ICI-therapy and in patients with higher tumor response. However, they are not the most frequent irAE after ICI exposure: 9.3% of all irAE were rheumatic (20 rh-irAE cases in 19 patients of a total of 215 irAE cases in 163 patients). As the use of ICIs is increasing for different malignancies the incidence of rh-irAE can be expected to increase.
References
1. Ribas A et al. Cancer immunotherapy using checkpoint blockade. Science. 2018
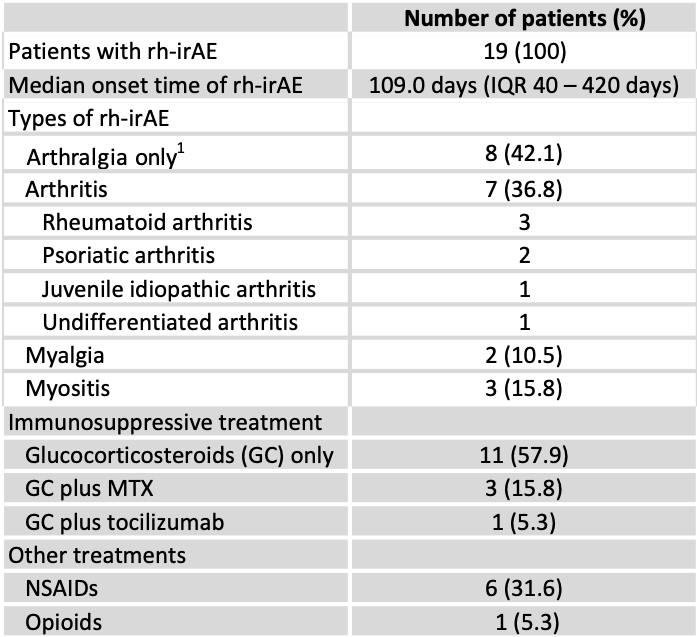 Table 1: Clinical characteristics of rheumatic immune-related adverse events (rh-irAE). 1= Excluding patients with arthritis. IQR: interquartile range, MTX: methotrexate, NSAIDs: non-steroidal anti-inflammatory drugs
Table 1: Clinical characteristics of rheumatic immune-related adverse events (rh-irAE). 1= Excluding patients with arthritis. IQR: interquartile range, MTX: methotrexate, NSAIDs: non-steroidal anti-inflammatory drugs
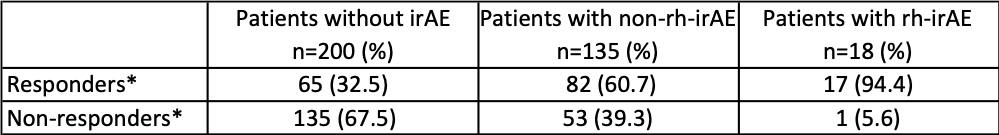 Table 2: Comparison of best tumor response to immune checkpoint inhibitors (ICIs) in patients without immune-related adverse events (irAE), patients with non-rheumatic irAE (non-rh-irAE) and in patients with rheumatic irAE (rh-irAE). *Response to ICI-therapy includes complete response, partial response and stable disease. Non-responders were affected by progressive disease. Patients who suffered from rh-irAE and non-rh-irAE were classified as rh-irAE cohort.
Table 2: Comparison of best tumor response to immune checkpoint inhibitors (ICIs) in patients without immune-related adverse events (irAE), patients with non-rheumatic irAE (non-rh-irAE) and in patients with rheumatic irAE (rh-irAE). *Response to ICI-therapy includes complete response, partial response and stable disease. Non-responders were affected by progressive disease. Patients who suffered from rh-irAE and non-rh-irAE were classified as rh-irAE cohort.
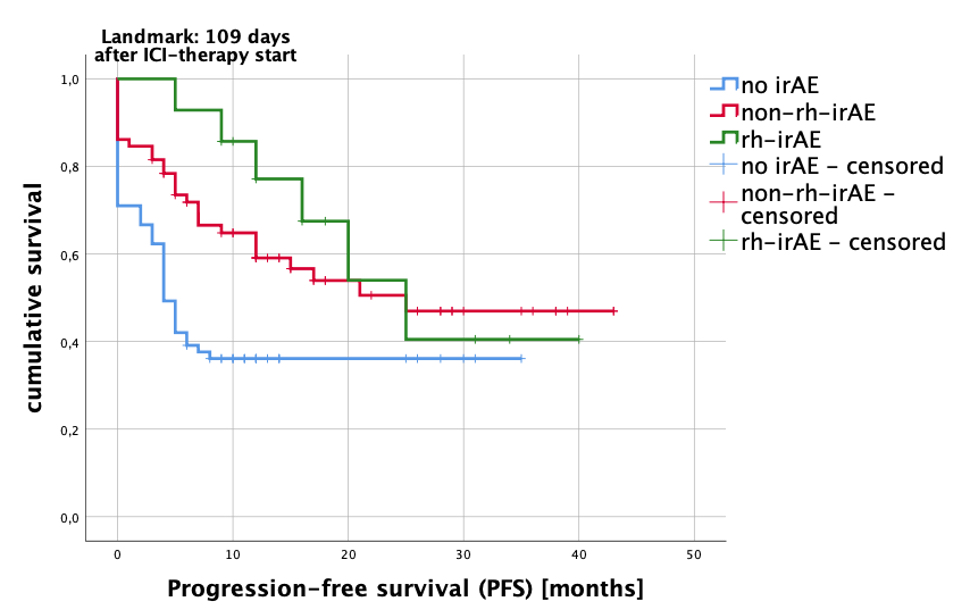 Fig 1: Kaplan-Meier analysis estimated PFS (progression-free survival) after immune checkpoint inhibitor (ICI) therapy. Compared were patients with no side effects (no irAE=blue line), patients with other non-rheumatic immune-related adverse events (non-rh-irAE=red line) and patients with rheumatic immune-related adverse events (rh-irAE=green line). Patients who suffered from rh-irAE and non-rh-irAE were classified as rh-irAE cohort. Patients with a therapy duration of 109 days or more were included in the analysis (landmark 109 days after ICI-therapy start). Log rank test: p=0.003.
Fig 1: Kaplan-Meier analysis estimated PFS (progression-free survival) after immune checkpoint inhibitor (ICI) therapy. Compared were patients with no side effects (no irAE=blue line), patients with other non-rheumatic immune-related adverse events (non-rh-irAE=red line) and patients with rheumatic immune-related adverse events (rh-irAE=green line). Patients who suffered from rh-irAE and non-rh-irAE were classified as rh-irAE cohort. Patients with a therapy duration of 109 days or more were included in the analysis (landmark 109 days after ICI-therapy start). Log rank test: p=0.003.
To cite this abstract in AMA style:
Verspohl S, Holderried T, Behning C, Brossart P, Schaefer V. Prevalence, Therapy and Tumor Response in Patients with Rheumatic Immune-related Adverse Events Following Immune Checkpoint Inhibitor Therapy: A Single-Centre Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/prevalence-therapy-and-tumor-response-in-patients-with-rheumatic-immune-related-adverse-events-following-immune-checkpoint-inhibitor-therapy-a-single-centre-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-therapy-and-tumor-response-in-patients-with-rheumatic-immune-related-adverse-events-following-immune-checkpoint-inhibitor-therapy-a-single-centre-analysis/
