Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Uveitis is the most common extra-articular manifestation of juvenile idiopathic arthritis (JIA) and can lead to vision loss if not detected early. Current clinical risk factors are insufficient for the reliable prediction of uveitis development. Tear fluid represents a non-invasive source for biomarker discovery. In this prospective study, we investigated tear-based biomarkers to enhance early prediction and monitoring of uveitis in patients with JIA.
Methods: This prospective multicenter study included children with JIA without uveitis (JIA-no-U) diagnosed within the first 4 years of JIA and JIA-associated uveitis (JIA-U) from the Predicting Eye Disease in Childhood Arthritis – Uveitis Study (PEDIA-U) cohort. Tear samples were collected from both eyes by Schirmer strips at baseline (BL) and at 4 follow-up visits (FU1-4), approximately every 3 months. Levels of s100A8/A9 and A12 were measured by ELISA; IL-8, CXCL10, MCP-1, sICAM-1, APRIL and VEGF-A by Luminex assays. Biomarker levels between JIA-U and JIA-no-U were compared at 1) BL and 2) longitudinally, including FU1 and FU2 visits only. The log-transformed data were used in mixed model analysis with individuals and eyes as random effects, adjusting for arthritis activity and testing for a time-by-uveitis status interaction for repetitive sample collection. Least square (LS) means comparisons are adjusted for multiple testing using the Tukey-Kramer method. Multivariate analysis was performed using PROC GLIMMIX.
Results: Forty-two patients (31 JIA-no-U and 11 JIA-U) contributed 142 tear samples (102 JIA-no-U and 40 JIA-U) (Table 1). All patients had a BL visit and 17 had at least one FU. Of 11 JIA-U patients, 2 had active and 9 had inactive uveitis at BL; two JIA-no-U patients developed uveitis during FU. Only two patients changed their uveitis activity status over time. At baseline, MCP-1 and CXCL-10 levels were significantly decreased in the JIA-U group compared to JIA-no-U (LS mean MCP-1: 1.31 vs. 2.18, p=0.008; CXCL-10: 4.01 vs. 5.1, p=0.001) (Figure 1). Over time, MCP-1 levels in the JIA-U group significantly increased from BL to FU2 (LS mean: BL = 1.2, FU = 3.1, p=0.027), while no significant change was observed in JIA-no-U. In contrast, CXCL-10 levels significantly decreased in JIA-no-U group from BL to FU2 (LS mean: BL = 4.9, FU = 4.0, p=0.0002), with no distinct change in JIA-U. For two patients who developed uveitis during FU visits, their MCP-1 and CXCL-10 levels followed the same trajectory as JIA-U group. In multivariate analysis, we found that levels of CXCL-10 (p=0.0036) and APRIL (p=0.026) were significantly associated with JIA-U diagnosis, and MCP-1 (p=0.008) showed a trend to significance. Additionally, S100A12 and VEGF-A significantly increased from BL to FU for both JIA-U and JIA-no-U groups (p=0.024, p=0.044, respectively). These changes might be related to JIA progression itself.
Conclusion: This is the first prospective study evaluating tear-based biomarkers over time in JIA. We found a distinct difference in MCP-1 and CXCL10 levels in tear fluid between JIA-U and JIA-no-U patients at BL and FU. This data might be used for early uveitis prediction in JIA patients and potential treatment enhancement.
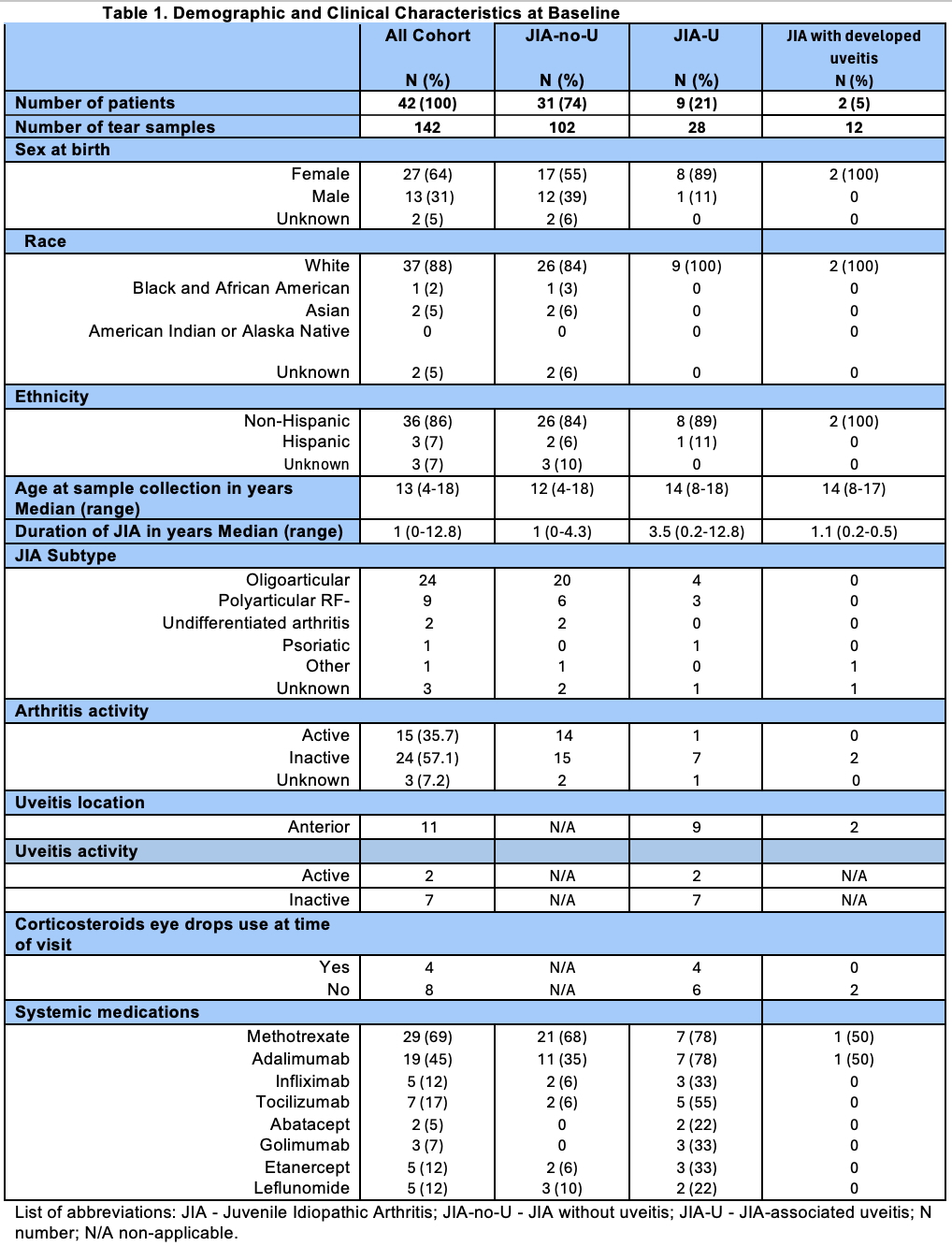 Table 1. Demographic and Clinical Characteristics at Baseline
Table 1. Demographic and Clinical Characteristics at Baseline
.jpg) Figure 1. Predicted means of MCP-1 and CXCL10 (IP-10) by group and time
Figure 1. Predicted means of MCP-1 and CXCL10 (IP-10) by group and time
To cite this abstract in AMA style:
Pavlenko M, Altaye M, Brunner H, Chang M, Cooper A, Davidson S, Duell A, Gangwani B, Hersh A, Holland G, Langefeld C, Lerman M, Lo M, Miraldi Utz V, Prahalad S, Schulert G, Quinlan-Waters M, Stahl E, Tsui E, Angeles-Han S. Predicting JIA-Associated Uveitis Using Tear Fluid Biomarkers: A Prospective Multicenter Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/predicting-jia-associated-uveitis-using-tear-fluid-biomarkers-a-prospective-multicenter-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicting-jia-associated-uveitis-using-tear-fluid-biomarkers-a-prospective-multicenter-study/
