Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Post-translational modifications with malondialdehyde-acetaldehyde (MAA) alter protein structure and function, inducing pro-inflammatory and pro-fibrotic responses characteristic of rheumatoid arthritis (RA). Matrix-gla protein (MGP) chelates intracellular calcium, thus potentially regulating function of calcium-dependent signal transduction leading to inflammation. As MAA strongly co-localizes with MGP in RA synovial tissues (Tieliwaerdi ACR 2020 abstract #0789), we have hypothesized that MGP is expressed by activated fibroblasts and that MAA-modification of this protein may increase the pool of intracellular calcium leading to production of pro-inflammatory cytokines.
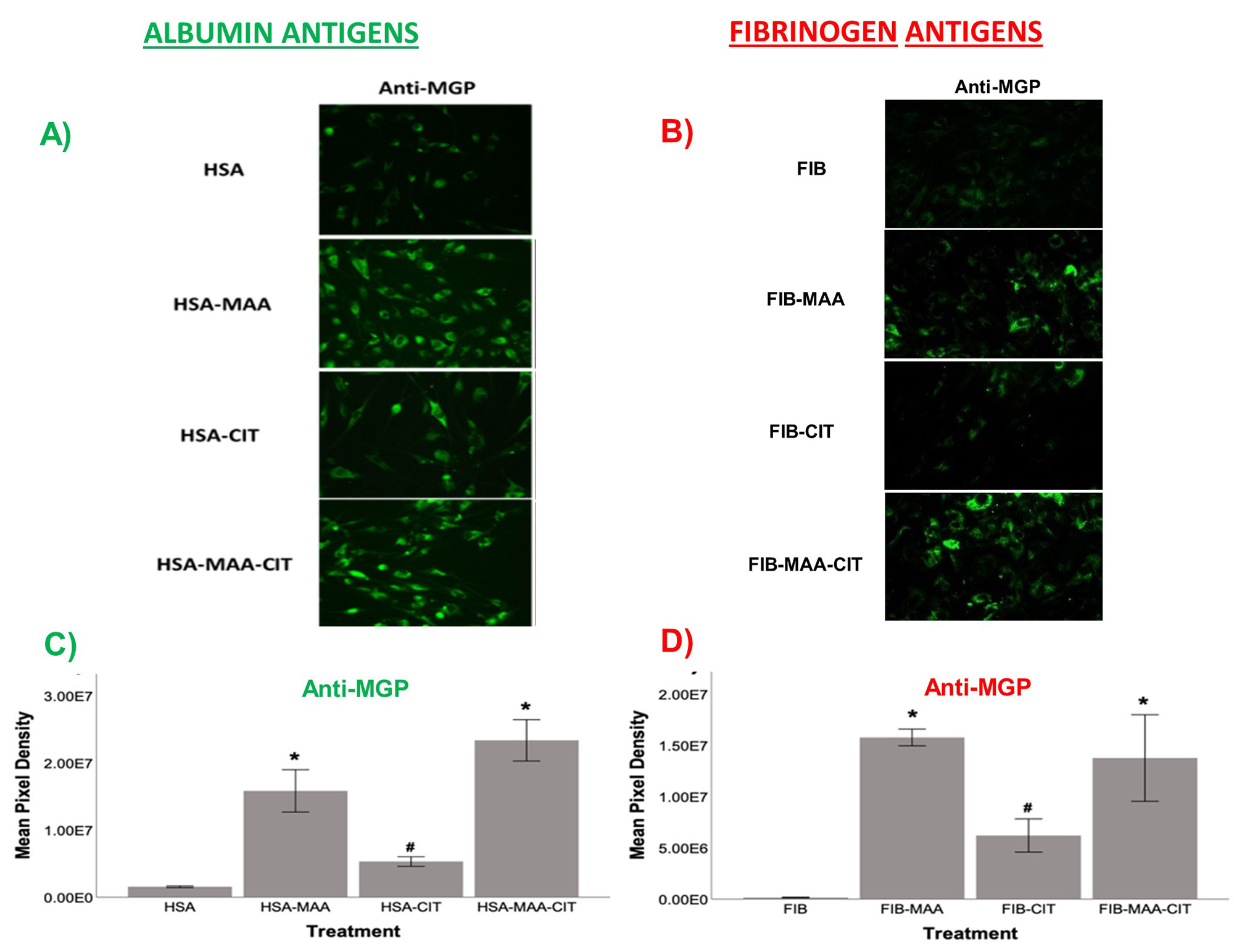
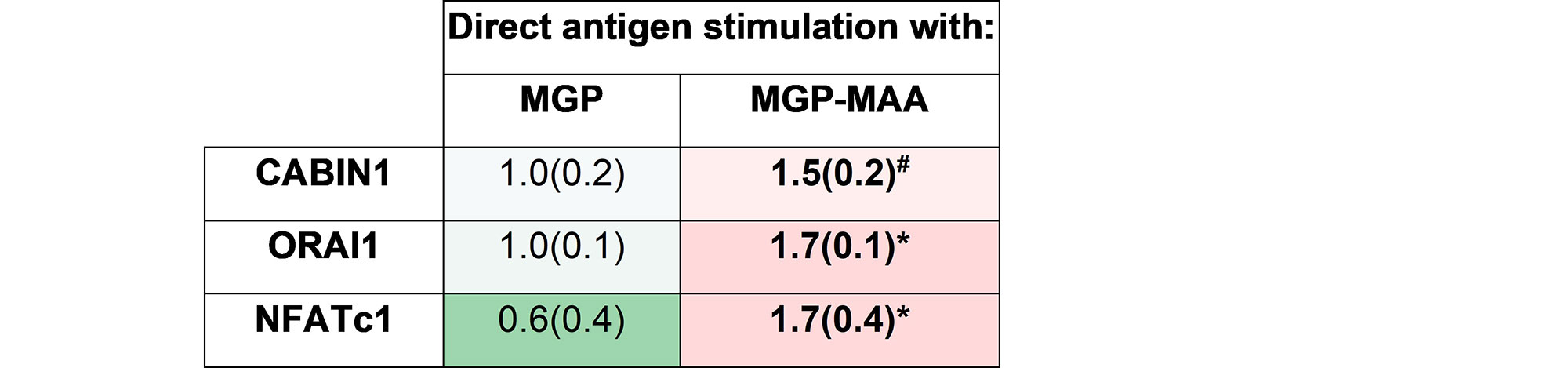
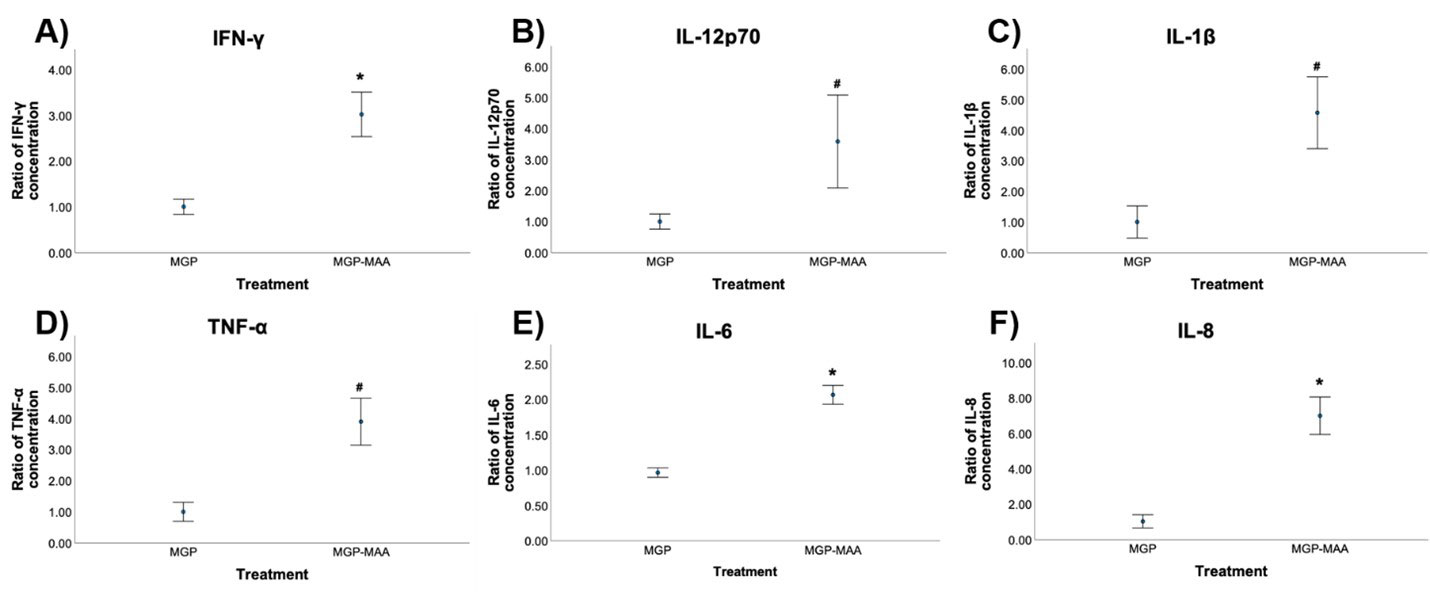
Methods: As prior experiments have shown that stimulation with post-translationally modified proteins leads to fibroblast activation, HFLS-RA cells were stimulated with human fibrinogen (FIB) or human serum albumin (HSA) that were MAA-modified (FIB-MAA/HSA-MAA), citrullinated (FIB-CIT/HSA-CIT), or dually MAA- and CIT-modified (FIB-MAA-CIT/HSA-MAA-CIT). HFLS-RA cells were stained and analyzed for MGP expression using fluorescent immunohistochemistry (IHC) and quantified as the mean pixel density using ImageJ. HFLS-RA cells were then stimulated with MGP or MGP-MAA for 48 hours, mRNA isolated and RT-PCR performed for calcium signaling molecules: CABIN1, ORAI1 and NFATc1. Release of pro-inflammatory cytokines in HFLS-RA supernatants were measured by ELISA following stimulation with MGP vs. MGP-MAA.
Results: MGP expression was significantly increased in HFLS-RA cells following stimulation with all modified forms of FIB and HSA vs. native proteins (Figure 1). Additionally, MGP-MAA stimulated HFLS-RA cells significantly upregulated mRNA levels of all calcium-signaling proteins: CABIN1 (1.5-fold, p< 0.05), ORAI1 (1.7-fold, p< 0.001), and NFATc1 (1.7-fold, p< 0.001) (Figure 2) compared to cells stimulated with native MGP. Pro-inflammatory cytokine concentrations were increased in HFLS-RA supernatants following stimulation with MGP-MAA vs. native MGP (Figure 3).
Conclusion: IHC results demonstrate that HFLS-RA indeed express and upregulate MGP in response to MAA and/or CIT modified antigens. Moreover, MAA-modified MGP leads to the increased expression of CABIN1, which responds to changes in calcium, thereby increasing ORAI1 to open calcium channels and activate NFATC1 to signal the release of pro-inflammatory cytokines. Taken together, these findings suggest that MAA modifications act as a regulator of MGP function and potentiate calcium-dependent pathways leading to inflammation. Ongoing studies are focused on determining if this increase in cellular calcium also increases the citrullination of self-proteins via calcium-dependent pepitidylarginine deiminase (PAD) and the generation of autoantigens implicated in the pathogenesis of RA.
To cite this abstract in AMA style:
Ragland A, Duryee M, Aripova n, Jones S, Mikuls T, Thiele G. Post-translational Modification of Matrix-gla Protein with Malondialdehyde-Acetaldehyde Alters Cellular Responses by Human Fibroblasts [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/post-translational-modification-of-matrix-gla-protein-with-malondialdehyde-acetaldehyde-alters-cellular-responses-by-human-fibroblasts/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/post-translational-modification-of-matrix-gla-protein-with-malondialdehyde-acetaldehyde-alters-cellular-responses-by-human-fibroblasts/