Session Information
Date: Monday, October 27, 2025
Title: (0955–0977) Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: SSc is a rare, chronic, systemic autoimmune rheumatic disease characterized by progressive skin and internal organ fibrosis, severe vasculopathy and immune dysregulation with production of specific autoantibodies. SSc manifestations such as skin thickening, interstitial lung disease (ILD), pulmonary arterial hypertension (PAH), and cardiac and gastrointestinal complications significantly impact patient quality of life. SSc pathophysiology is complex, but the association between autoantibodies and disease subtype, disease course, and occurrence of specific disease manifestations suggests a role in SSc pathophysiology. Moreover, several different autoantibodies have been reported that can directly promote inflammation and/or fibrosis. Currently approved therapies are at best slowing down disease progression, underscoring a high medical need for novel therapies.
Methods: Efgartigimod, a human IgG1-derived Fc fragment, is engineered to block the neonatal Fc gamma receptor (FcRn), enhancing IgG degradation and reducing circulating disease-causing IgG autoantibodies (Figure 1). Efgartigimod is approved for the treatment of generalized myasthenia gravis and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). We evaluated the effects of FcRn blockade in a topoisomerase I (topo I)-induced mouse model of SSc, which recapitulates the autoimmune, inflammatory, and fibrotic features of the disease.
Results: Treatment with the murine homologue of efgartigimod (an IgG-derived Fc fragment targeting mouse FcRn) was well tolerated. Both prophylactic (treatment started before the onset of immunization) and therapeutic (treatment 2 weeks after immunization with human topo I-antigen) administration of the murine homologue of efgartigimod significantly ameliorated dermal fibrosis and interstitial lung disease as evidenced by reduced dermal thickness, Ashcroft scores, myofibroblast number, and collagen content. Notably, the antifibrotic effects were comparable to those of nintedanib. Gene expression analysis in mouse skin revealed regulatory effects of the murine homologue of efgartigimod on inflammatory/autoimmune and fibrotic signaling pathways with inhibitory effects on CCL-chemokines, interleukins, S100A4, and on transforming growth factor β and JAK/STAT signaling. In addition, treatment with the FcRn blocker improved gastrointestinal transit that was delayed in mice immunized with topo I.
Conclusion: These findings highlight that, in a murine model of SSc, FcRn blockade may have potential to simultaneously target the autoimmune, inflammatory and fibrotic features of SSc and highlight the potential of autoantibody-reducing therapies as disease-modifying approaches in this disease. Indeed, a randomized, controlled phase II trial with efgartigimod in SSc is currently ongoing.
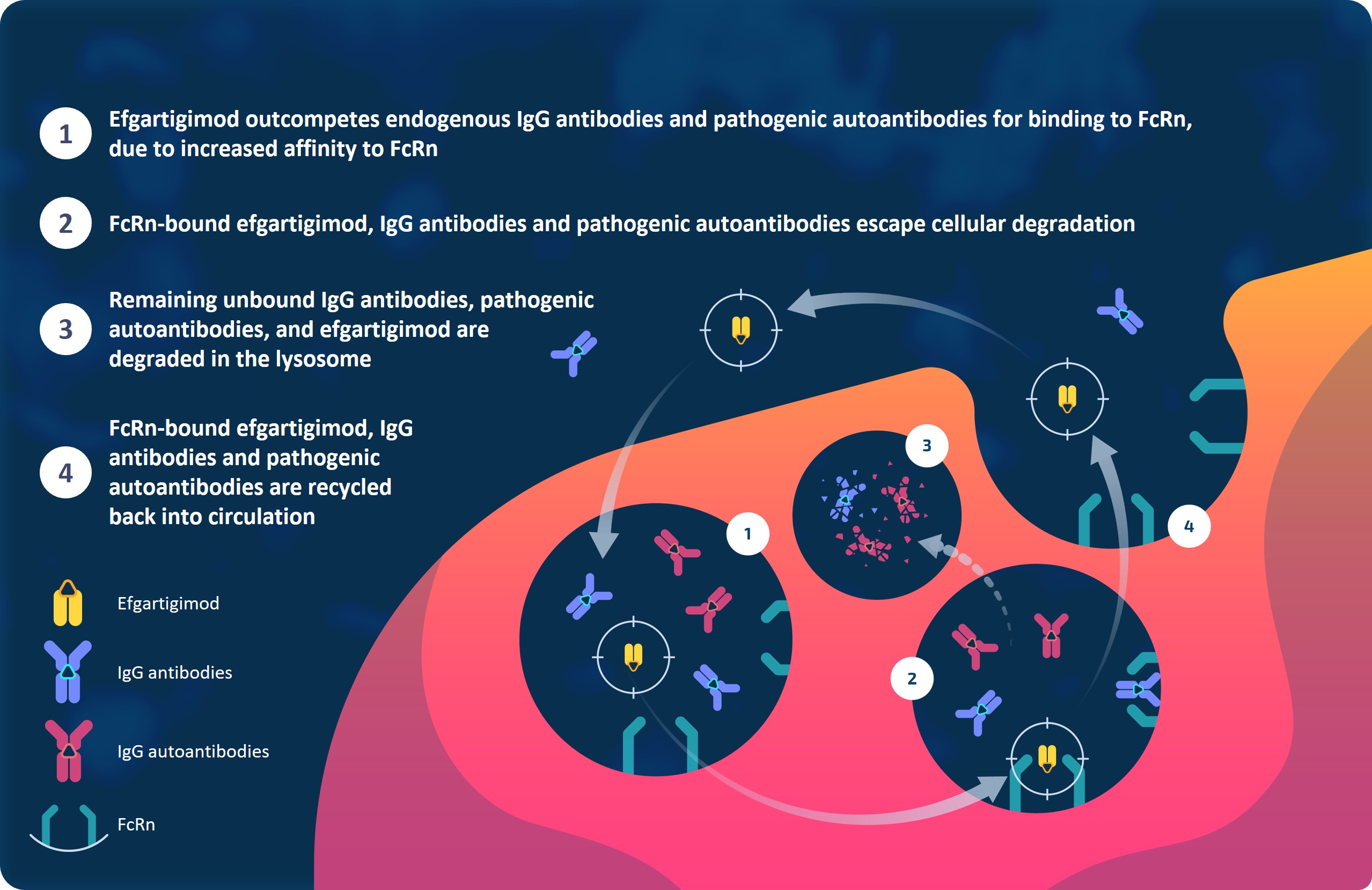 Figure 1. Mechanism of action of efgartigimod against IgG-mediated fibrosis.
Figure 1. Mechanism of action of efgartigimod against IgG-mediated fibrosis.
To cite this abstract in AMA style:
Tran-Manh C, Trinh-Minh T, Liebel C, van der Woning B, Zabrocki P, Distler J. Pharmaceutical Blockade of the Neonatal Fc Gamma Receptor Ameliorates Autoimmunity, Inflammation and Fibrosis in the Topoisomerase I Mouse Model of Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/pharmaceutical-blockade-of-the-neonatal-fc-gamma-receptor-ameliorates-autoimmunity-inflammation-and-fibrosis-in-the-topoisomerase-i-mouse-model-of-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmaceutical-blockade-of-the-neonatal-fc-gamma-receptor-ameliorates-autoimmunity-inflammation-and-fibrosis-in-the-topoisomerase-i-mouse-model-of-systemic-sclerosis/
