Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Physicians often consider adverse events when choosing therapies for PsA and RA but may give less attention to other ways in which treatments affect the well-being of patients, such as impacts on quality of life, barriers to access, and challenges with taking medications. In a previous study examining the prevalence of side effects to TNF inhibitors (TNFi) and methotrexate (MTX) in FORWARD, The National Databank for Rheumatic Diseases, we found that side effects were wide ranging and differed for PsA and RA and by treatment. The objective of this qualitative study was to consider patients’ perspectives on the treatment burdens of MTX and TNFi’s, the most commonly used therapies in PsA and RA.
Methods: We conducted semi-structured interviews with 24 patients with RA and 25 patients with PsA from 05/2019-03/2020 who were enrolled in FORWARD with documented use of MTX or TNFi. Participants were asked about their experiences with medications for their disease, specifically MTX and TNFi. Interviews were recorded and transcribed. Transcripts were analyzed using a grounded theory approach and NVivo software (v12.0). We coded concepts related to treatment burden, effectiveness of medications, decision-making, disease course, and impacts on daily life such as social relationships and work. We examined how medication type shaped patient experiences with treatment burdens.
Results: Participants were mostly female (72% in PsA, 88% in RA), all Caucasian, aged 45-85 years, and with disease duration of 4-51 years (Table 1). All participants had used MTX and 82% had used TNFi. We identified 9 types of treatment burdens (Table 2): side effects, managing side effects, psychological, daily functioning, taking medications, accessing medications, economic impact, work, and family planning/breastfeeding. For both patients taking MTX and TNFi, fewer than half of patients reported increased infections/cancer or seeking/receiving further medical attention or care to address side effects. A majority of MTX users reported taking steps to manage the negative impacts of nausea, fatigue, mouth sores, or hair loss on quality of life, daily functioning or work. Three-quarters of TNFi users reported challenges with accessing, paying for, or taking medications. Consistent with previous research, discontinuing the use of medication because of lack of effectiveness was more common among participants with PsA than those with RA (data not shown).
Conclusion: Greater awareness of the treatment burdens experienced by patients may help healthcare providers better support patients with any medication-related challenges that they encounter. From the perspective of patients, we found that there are many treatment burdens associated with PsA and RA that are rarely addressed in clinical trials or observational studies.
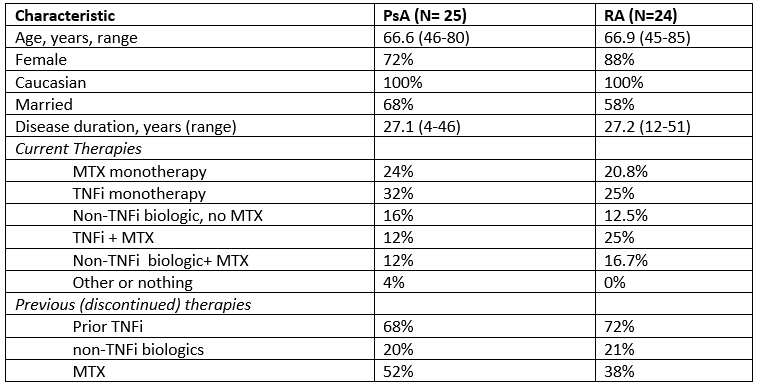 Table 1. Participant characteristics by diagnosis (mean (range) or %)
Table 1. Participant characteristics by diagnosis (mean (range) or %)
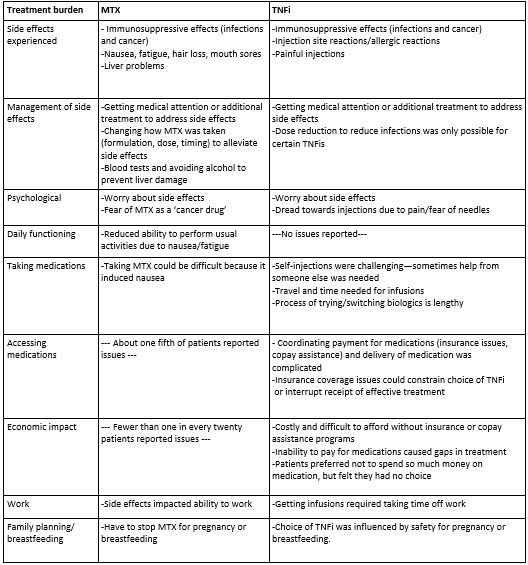 Table 2. Key Treatment Burdens Discussed by Participants
Table 2. Key Treatment Burdens Discussed by Participants
To cite this abstract in AMA style:
Ogdie A, Shaw Y, Almonte M, Maksabedian E, Michaud K. Perspectives on Treatment Burden for Methotrexate and TNF-inhibitors Among Psoriatic Arthritis and Rheumatoid Arthritis Patients: A Qualitative Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/perspectives-on-treatment-burden-for-methotrexate-and-tnf-inhibitors-among-psoriatic-arthritis-and-rheumatoid-arthritis-patients-a-qualitative-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/perspectives-on-treatment-burden-for-methotrexate-and-tnf-inhibitors-among-psoriatic-arthritis-and-rheumatoid-arthritis-patients-a-qualitative-study/
