Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical I: Treatment of JIA (1492–1496)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Juvenile idiopathic arthritis (JIA) patients may experience significant medication-related adverse effects (AEs), which may adversely affect health-related quality of life (HRQOL), daily activities and treatment compliance. The study aims to investigate Patient-Reported Adverse Events (AEs) and their effects on HRQoL, school activity and therapeutic adherence, as captured by patient- and parent-reported outcome measures (PROMs).
Methods: Data on 13704 visits of 8402 patients were obtained from two large multi-center international studies, the pharmacovigilance registry Pharmachild and The EPidemiology, treatment and Outcome of Childhood Arthritis (EPOCA) cohort. Subjects who were on medications at the time of visit were included. PROMs were collected through the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) and included checklists of AEs (18 items), disease-related school problems (5 items), self-reported treatment adherence (as a dichotomous variable) and reasons for non-compliance (5 items). Pain and overall well-being (PGA) were measured by 21-numbered circle visual analog scales. HRQoL was assessed through a ten-items Likert-type HRQoL scale encompassing a physical health (PhH) and psychosocial health (PsH) subscale, with higher scores indicating worse outcomes. The effects of AEs on PGA, PsH scale, school problems, and self-reported non-adherence were analyzed using General Linear and Generalized Mixed Effects Models with random intercepts per individual.
Results: AEs were reported by 29,49% of patients. Frequencies of individual AEs are shown in Fig.1. Experiencing one or more AEs was associated to worse PGA (OR 1,46 95%Cl 1.38-1.45, η2 0.011, p < .001) and PsH score (OR 1.85, 95%Cl 1.72-2.00, η2 0.024, p < .001) and school problems (OR 1.82, 95%Cl 1.64-2.01, p < .001) after adjustment for physician global assessment, PhH and pain levels. The effects of individual AEs on outcomes are reported in Fig. 2; mood swings, sleep problems and weight gain showed the highest impact on psychosocial health. Treatment non-adherence was reported by 9,27% of subjects; the most frequently cited reasons were drug refusal by the child (15,4%) and fear of adverse events (10,9%). Subjects reporting more than two AEs had a higher risk of non-adherence (OR 1.47, 95%Cl 1.21 - 1.76, p < .001). Nausea was associated with non-compliance specifically due to fear of AEs, as shown in Fig. 3 (OR 4.57, 95%Cl 2.98-6.92, p < .001).
Conclusion: AEs have a substantial impact on patients’ quality of life, functioning and treatment adherence in JIA. The understanding treatment-related burden is vital to achieve good therapeutic compliance and improve outcomes in JIA.
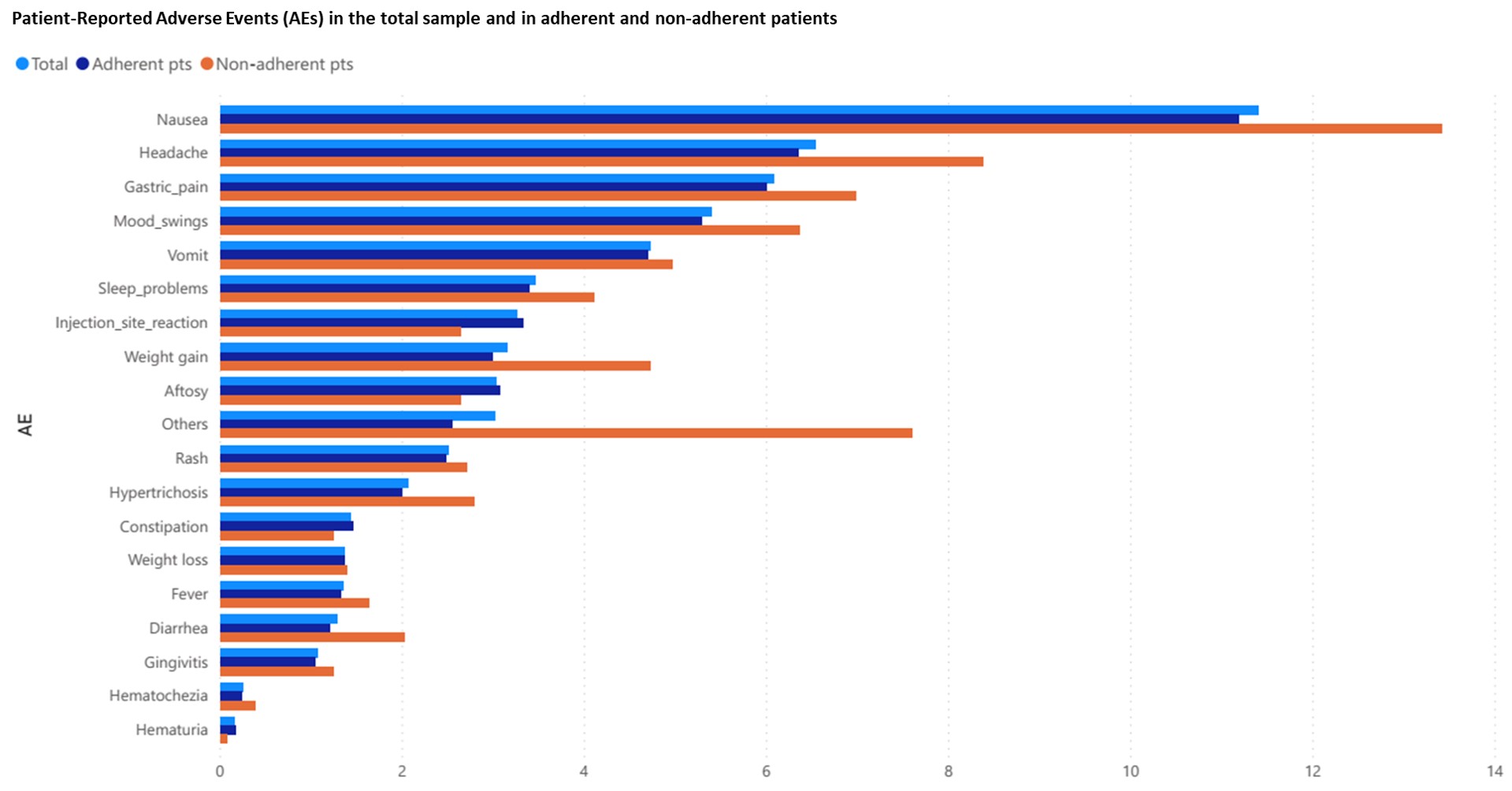 Fig. 1 – Frequencies (percentages) of Patient-Reported Adverse Events (AEs) in the total sample (light blue), adherent (dark blue) and non-adherent patients (orange).
Fig. 1 – Frequencies (percentages) of Patient-Reported Adverse Events (AEs) in the total sample (light blue), adherent (dark blue) and non-adherent patients (orange).
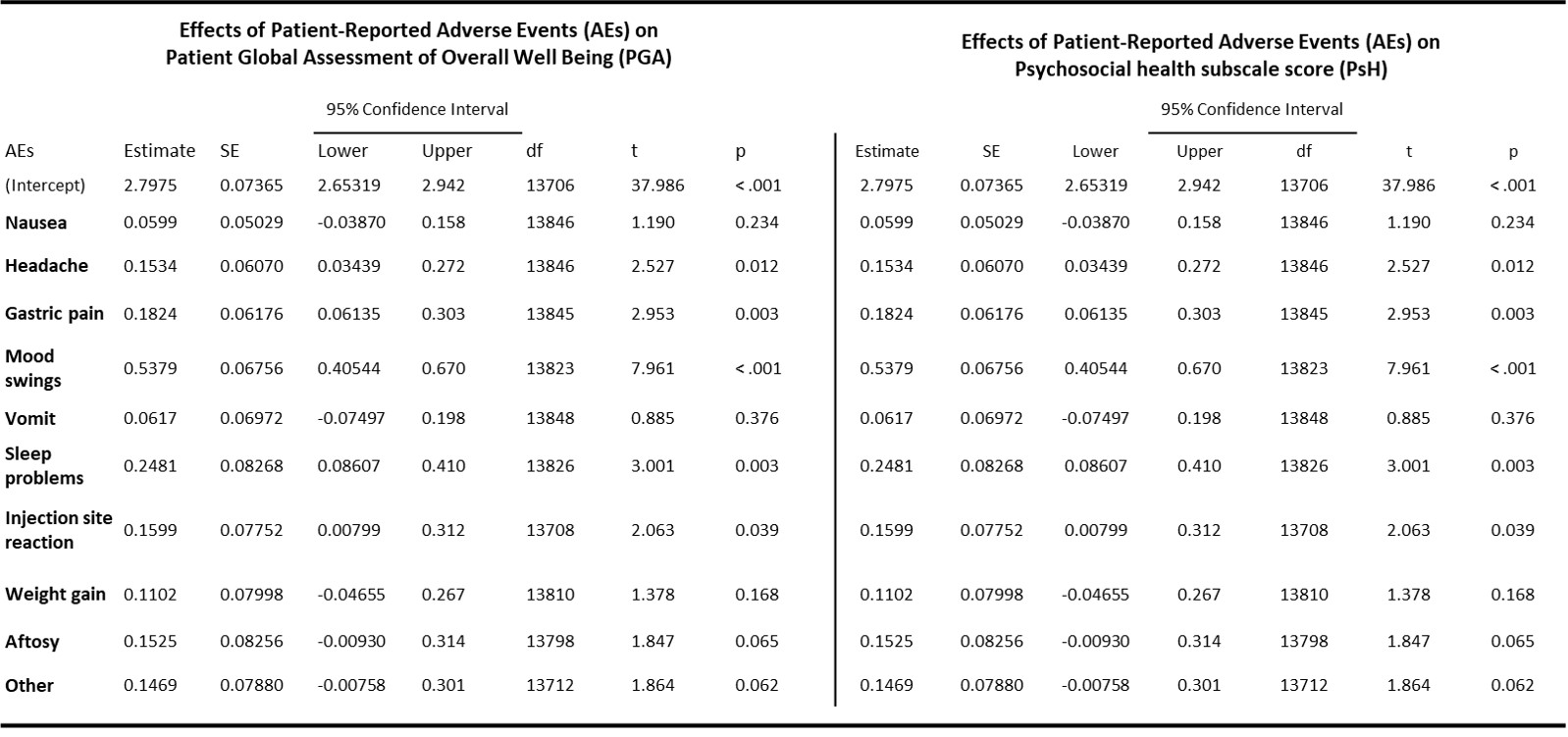 Fig. 2 – Results from linear mixed-effects regression models show the effects of the most commonly reported AEs (frequency > 3%) on Patient Global Assessment (PGA) and Psychosocial Health subscale score (PsH). The model for PGA is adjusted for pain levels and physician global assessment; the model for PsH is adjusted for physical health quality of life as measured by Physical Health subscale score (PhH).
Fig. 2 – Results from linear mixed-effects regression models show the effects of the most commonly reported AEs (frequency > 3%) on Patient Global Assessment (PGA) and Psychosocial Health subscale score (PsH). The model for PGA is adjusted for pain levels and physician global assessment; the model for PsH is adjusted for physical health quality of life as measured by Physical Health subscale score (PhH).
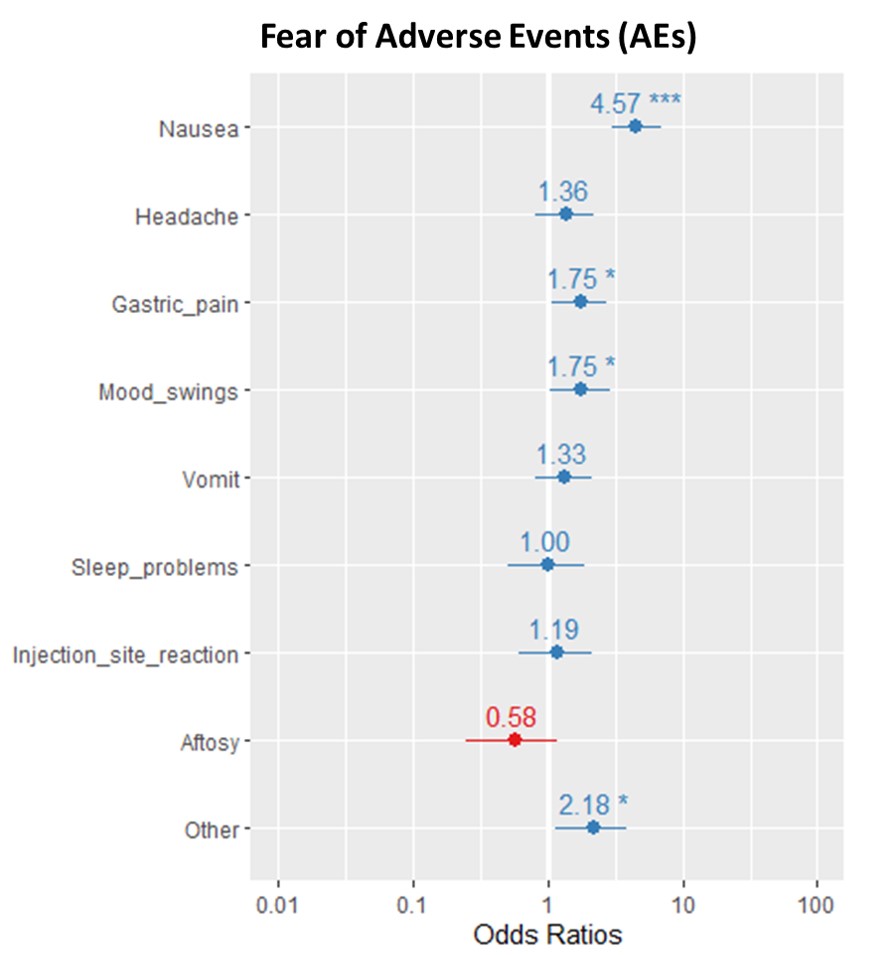 Fig. 3 – Risk (expressed as odds ratios) of self-reported non-adherence due to fear of Adverse Events (AEs) for individual AEs. * p ≤ 0.05, *** p ≤ 0.001
Fig. 3 – Risk (expressed as odds ratios) of self-reported non-adherence due to fear of Adverse Events (AEs) for individual AEs. * p ≤ 0.05, *** p ≤ 0.001
To cite this abstract in AMA style:
Alongi A, Trachana M, Stanevicha V, Ailioaie L, Tsitsami E, Ravelli A, Consolaro A, Ruperto N. Patient-Reported Adverse Events, Quality of Life and Treatment Adherence in Juvenile Idiopathic Arthritis: Analysis of Two Large International Cohorts [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/patient-reported-adverse-events-quality-of-life-and-treatment-adherence-in-juvenile-idiopathic-arthritis-analysis-of-two-large-international-cohorts/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-adverse-events-quality-of-life-and-treatment-adherence-in-juvenile-idiopathic-arthritis-analysis-of-two-large-international-cohorts/
