Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is a prototypical autoimmune disease where autoantibody production and immune complex (IC) formation play a key role. Which antibodies contribute to the load of circulating C1q-binding ICs (CIC) in SLE is still unclear. We investigated whether either serum and/or IC-derived levels of typical SLE antibodies could explain the observed CIC levels of a large SLE cohort.
Methods: We studied n=530 consecutive SLE patients (≥ 4 ACR 1982 revised SLE criteria) who received care at a tertiary referral center for SLE. The control population consisted of region of residence, age and sex-matched controls (n=192) and n=200 local experimental controls. Serum was obtained at inclusion, and was stored at -80°C. To obtain IC-derived antibodies, a novel validated method was employed. Briefly, aliquots of sera were incubated with C1q-coated beads and C1q-bound ICs were then eluted through two sequential buffers, yielding disassembled IC eluates with conserved antibody specificity (Sohrabian et al. Ann Rheum Dis 2018;77:1345). Antibody levels of Anti-dsDNA, Anti-Histone, Anti-Sm, Anti-U1RNP, Anti-Ro52, Anti-Ro60, Anti-SSB were measured in IC eluates and in serum with a bead-based multiplex assay. Parallel analyses of autoantibody levels in serum and in IC eluates were performed. CIC levels were measured with a commercial ELISA according to the manufacturer’s instructions. Results were reported on a standard curve; the 98th percentile of the standard curve values of control sera were set as the threshold for positivity in serum and IC respectively.
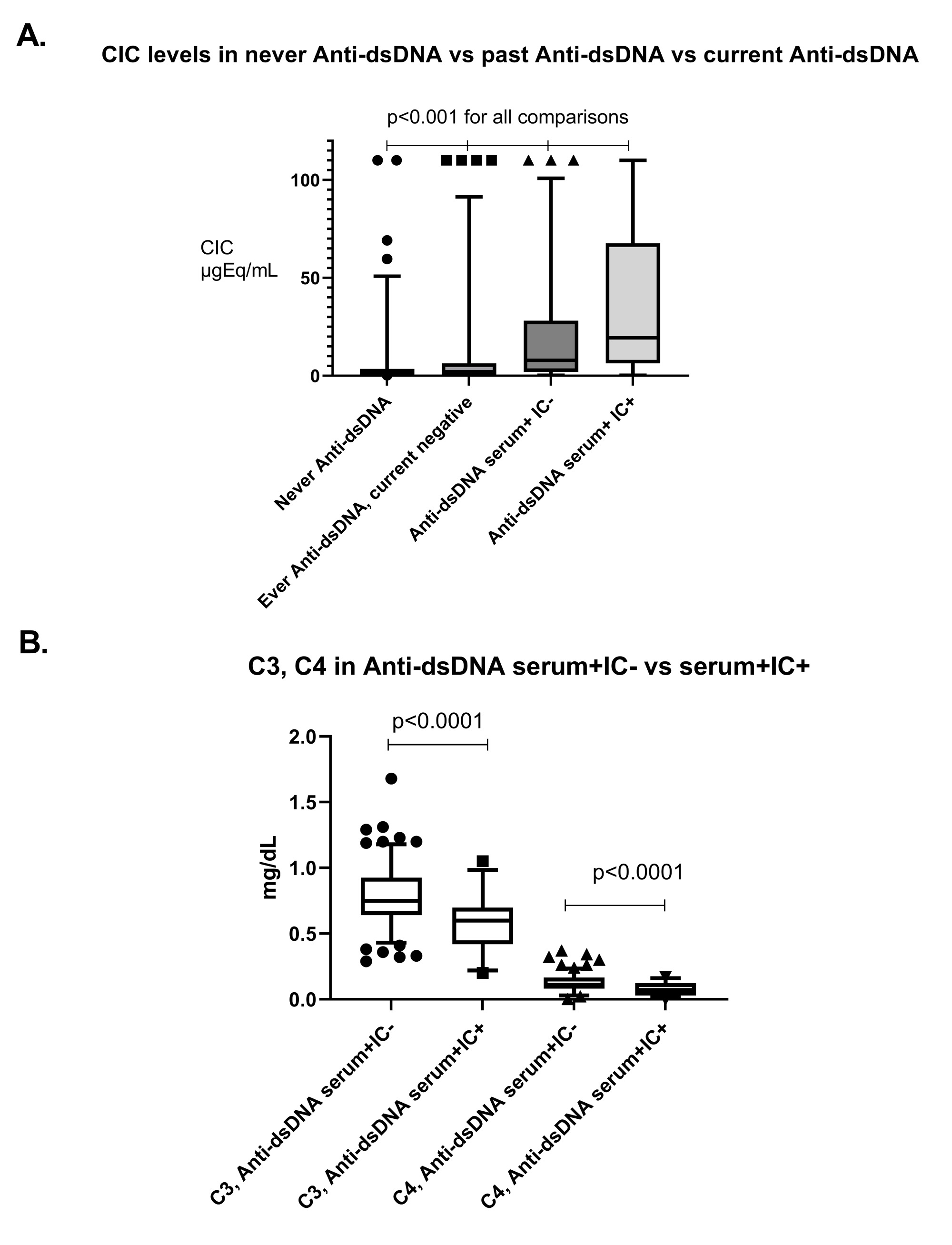
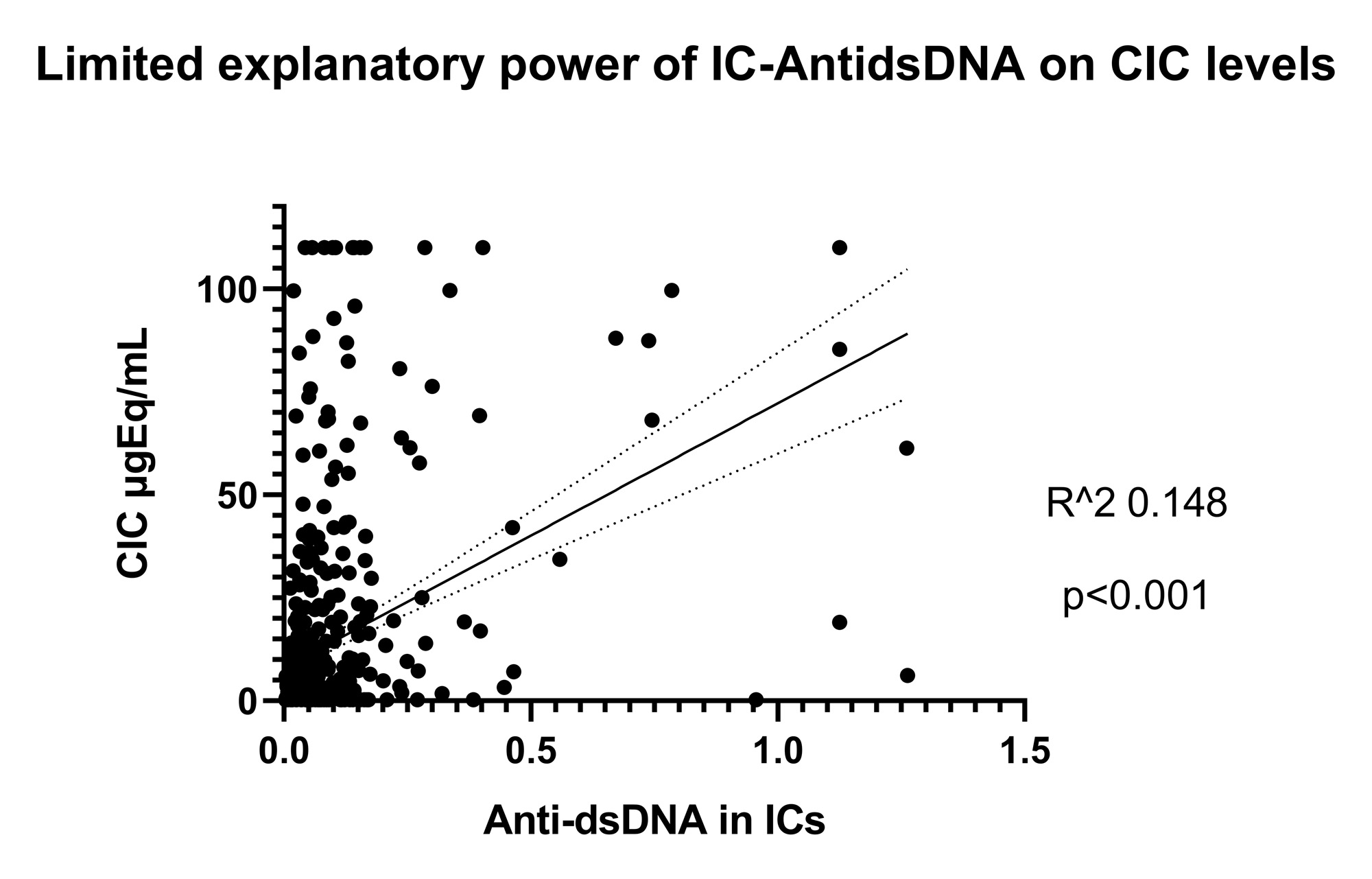
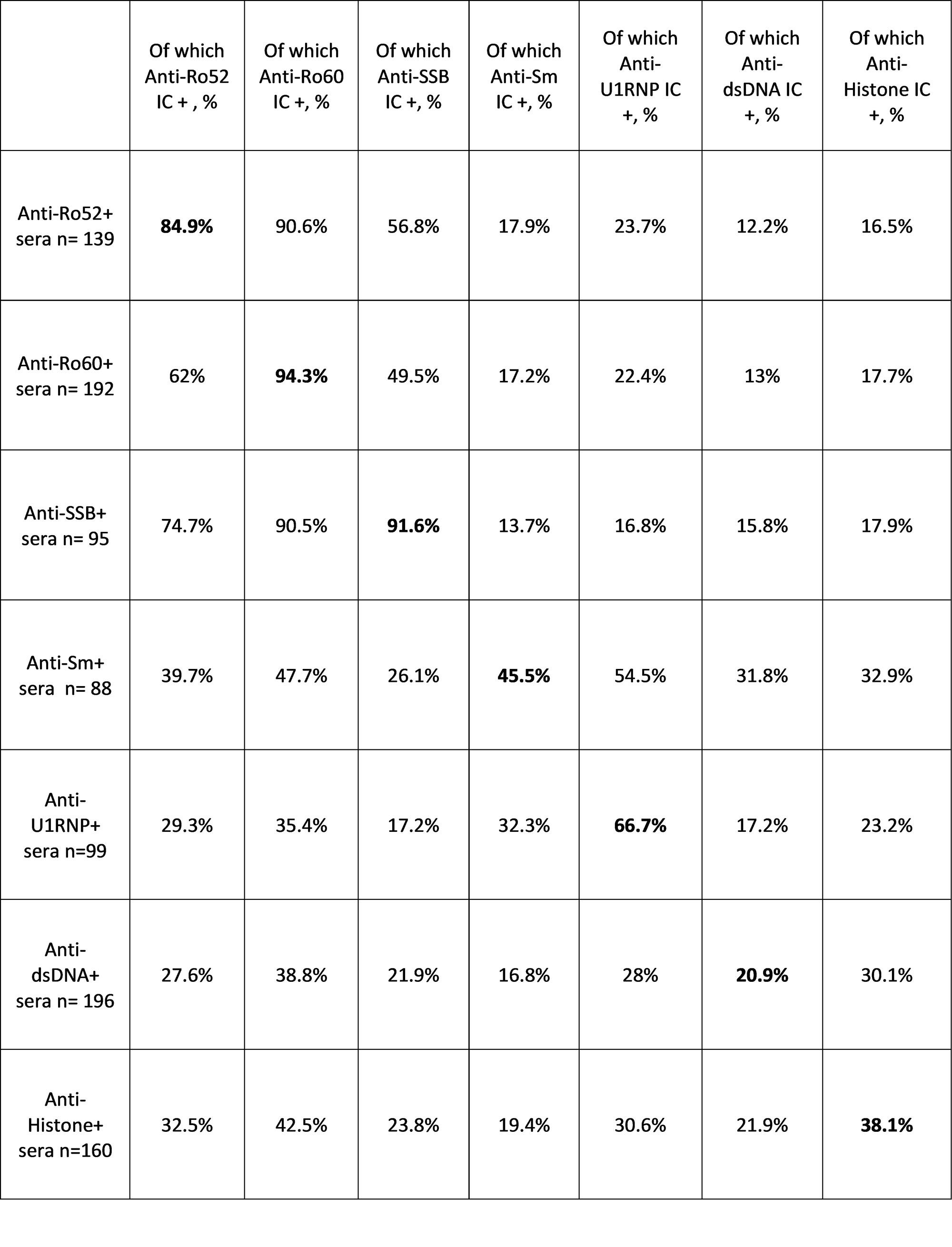
Results: CIC levels were higher and with a wider variance in SLE patients than controls (p< 0.0001). A higher CIC load was associated with nephritis, arthritis and SLEDAI > 6 (p< 0.0001). Among autoantibody specificities, Anti-dsDNA in serum was independently associated with a high CIC load (OR 4.8, 95% CI 2.5-9.5, p< 0.0001, Figure 1 A). Concordance between serum and IC-eluate positivity varied strongly among autoantibody specificities, being highest for Anti-SSA/SSB, and lowest for Anti-dsDNA (Table 1). Among serum Anti-dsDNA+ patients, carriers of Anti-dsDNA from ICs displayed higher Anti-dsDNA titers, lower complement levels, and higher SLEDAI scores (p< 0.01 for all, Figure 1 B). Despite this, anti-dsDNA from ICs had a limited, although significant, explanatory power for total CIC levels (Figure 2).
Conclusion: Anti-dsDNA indicates the capacity to assemble immune complexes in SLE patients, and recoverable Anti-dsDNA antibodies from IC eluates are associated with more complement activation and disease activity. Importantly, Anti-dsDNA, either alone or in combination with common ANA specificities, has a limited explanatory power for the variation in CIC levels among different SLE patients, i.e. it is unlikely to form the majority of the observable CIC in SLE. The constituents of the circulating immune complex load in SLE remain elusive and deserve further elucidation.
To cite this abstract in AMA style:
Fuzzi E, Svanqvist A, Westerberg C, Zickert A, Gunnarsson I, Rönnelid J, Svenungsson E. Paired Autoantibody Specificities from Serum and Immune Complexes Do Not Fully Explain the Circulating Immune Complex Load in Systemic Lupus Erythematosus: A Study on 530 Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/paired-autoantibody-specificities-from-serum-and-immune-complexes-do-not-fully-explain-the-circulating-immune-complex-load-in-systemic-lupus-erythematosus-a-study-on-530-patients/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/paired-autoantibody-specificities-from-serum-and-immune-complexes-do-not-fully-explain-the-circulating-immune-complex-load-in-systemic-lupus-erythematosus-a-study-on-530-patients/