Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Osteoarthritis (OA) results in joint pain, cartilage degeneration, and synovitis. Recent studies suggest that some OA patients are self-medicating with cannabis. Δ9-tetrahydrocannabinol (THC) is a prominent psychoactive phytocannabinoid in cannabis that can signal in joint cells, including chondrocytes and fibroblast-like synoviocytes (FLS). Currently, no studies link THC to disease modification in OA. We investigated effects and mechanisms of action of THC on pain and disease modification in pre-clinical models of knee OA.
Methods: OA was induced in male C57BL/6 mice by destabilization of the medial meniscus (DMM) or monosodium iodoacetate (MIA; 0.5 mg) and mice were administered THC (0, 1, 5 or 10 mg/kg) orally 5 days/week for 9 or 3 weeks, respectively. Von Frey tests were used to evaluate mechanical allodynia (pain) and open field tests were used to evaluate locomotion and anxiety. DMM knee joints were evaluated for cartilage degeneration/synovitis (OARSI scoring) and Ki67, αSMA, and collagen degradation (C2C) expression (immunohistochemistry). Human FLS and chondrocytes were cultured with 1 mM THC for 48h. RNA was sequenced to determine differentially expressed genes (DEGs; FDR-adjusted p< 0.05; absolute FC≥1.5) and DEG-enriched pathways. Pathway and transcription factor enrichment analyses were used to determine putative cellular mechanisms and transcriptional regulators modified by THC.
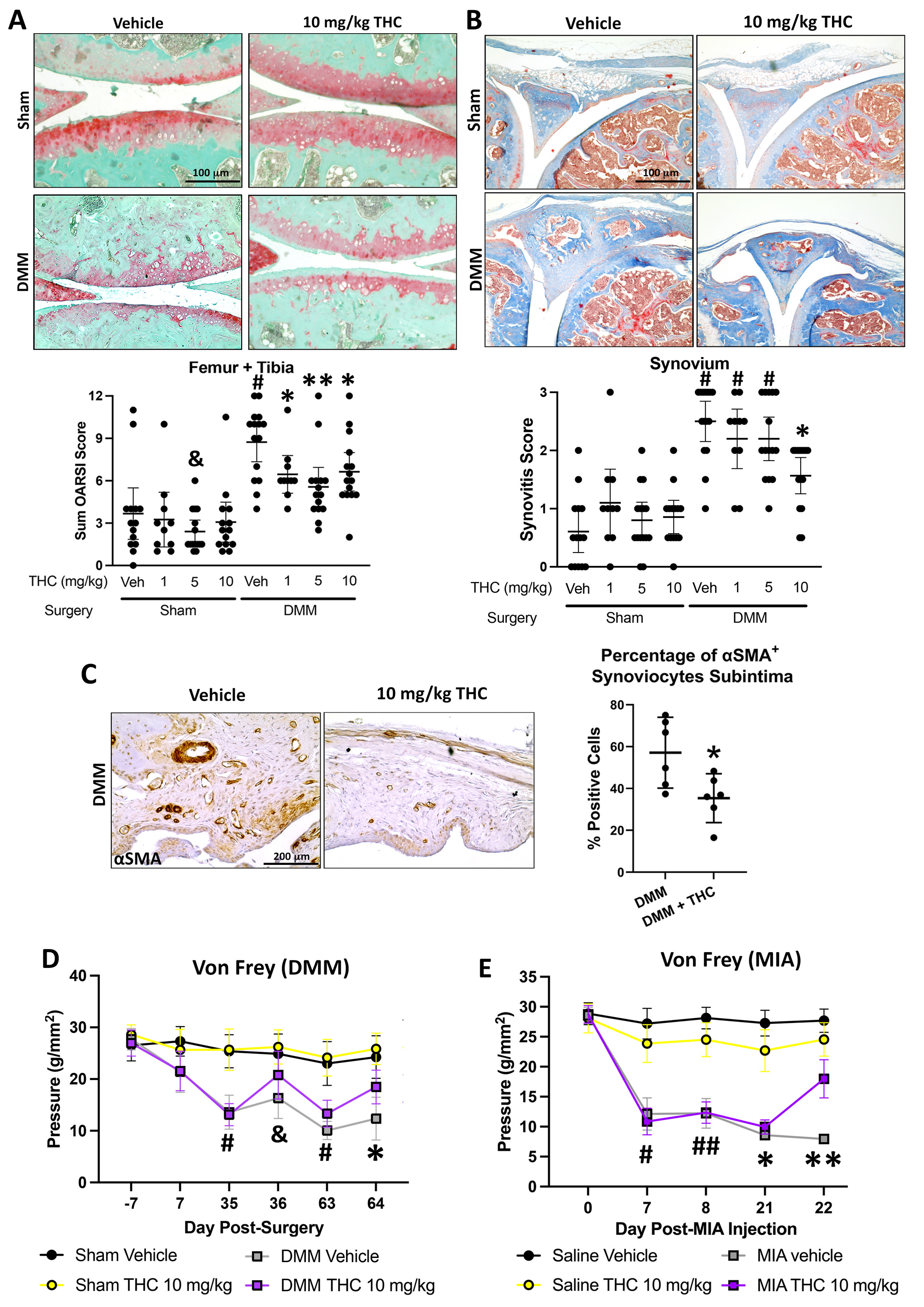
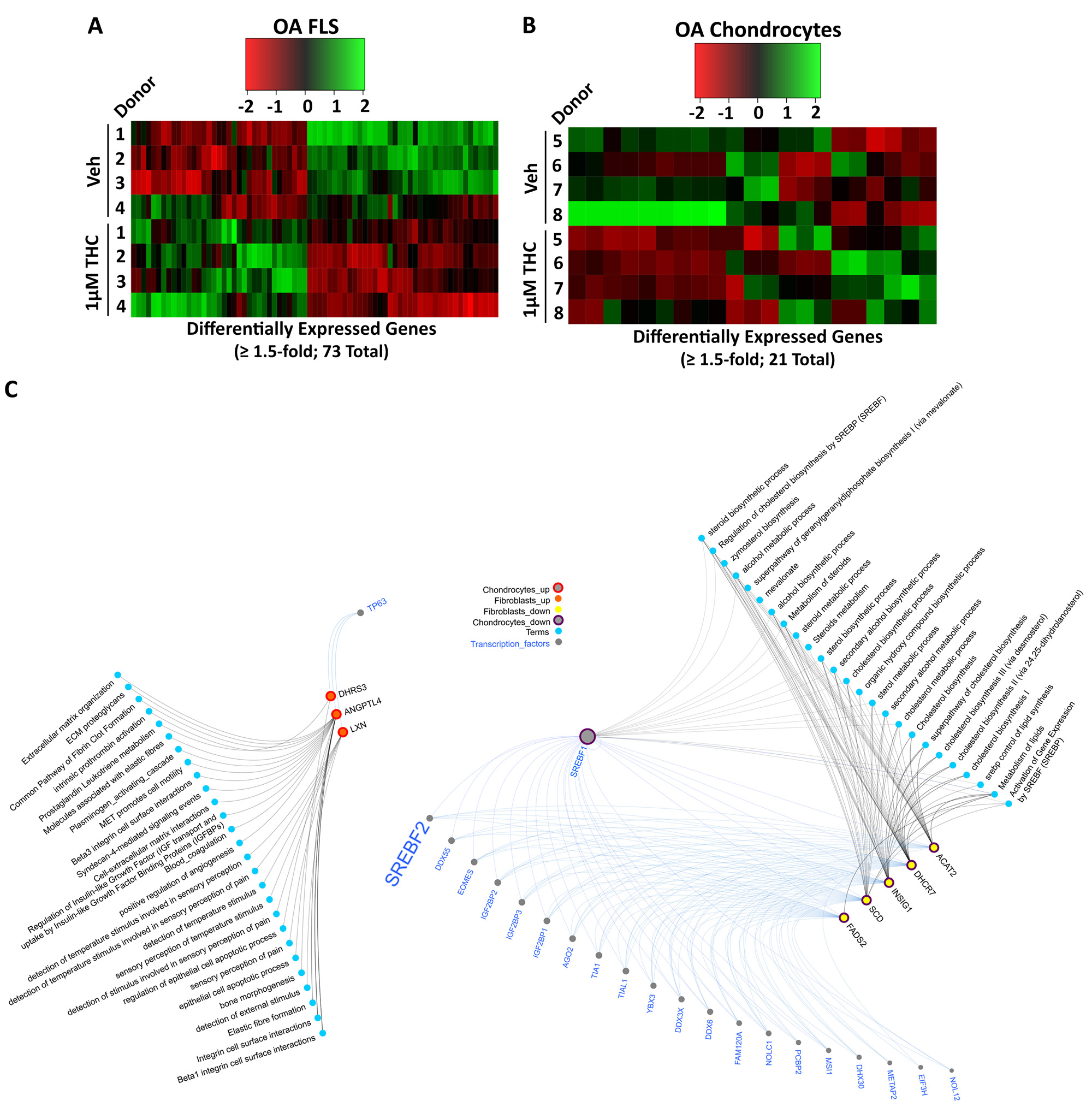
Results: In DMM mice, all THC doses reduced cartilage degeneration. 10 mg/kg THC also reduced synovitis (n=14-15/group) and synovial αSMA expression, but not Ki67 or C2C expression (n=6/group). Administration of 5 or 10 mg/kg THC in DMM (n=14-15/group) or MIA (n=10 25/group) mice reduced pain, particularly with 10 mg/kg administration (Fig. 1). Locomotion or anxiety were not modified longitudinally by THC administration. In cultured OA FLS (n=4), RNA sequencing identified 73 DEGs (35 up, 38 down) after treatment with 1 mM THC for 48h compared to vehicle. In cultured OA chondrocytes (n=4), 21 DEGs (9 up, 12 down) were identified after treatment with 1 mM THC compared to vehicle. Computational biology analyses found extracellular matrix (ECM)-related pathways were enriched in the upregulated DEGs in both fibroblasts and chondrocytes, while lipid/steroid/cholesterol-related pathways were enriched in the downregulated DEGs in both cell types. Three genes were commonly upregulated while 5 genes were commonly downregulated in FLS and chondrocytes by THC treatment. From FLS and chondrocyte downregulated DEGs, 22 common putative transcription factors were identified as potential regulators, with SREBF2 as a candidate of interest (Fig. 2).
Conclusion: Oral administration of 10 mg/kg THC reduced cartilage degeneration synovitis and synovial αSMA expression in DMM mouse knee joints, and modified pain in both DMM and MIA OA models. THC treatment of human OA FLS or chondrocytes modified expression of genes associated with ECM and lipid-based pathways, with common genes and putative regulatory transcription factors identified, including SREBF2. We are currently investigating contributions of SREBF2 to effects of THC on joint cells in vitro and mechanisms of pain in vivo.
To cite this abstract in AMA style:
rockel J, Maglaviceanu A, Fetter Filippini H, Wasilewski E, Lewis-Bakker M, Gabrial S, Rossomacha E, Garcia J, Mahomed N, Leroux T, Clarke H, Bonin R, Kotra L, Kapoor M. Oral Delivery of Δ9-Tetrahydrocannabinol Provides Symptom and Disease Modification in a Mouse Model of Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/oral-delivery-of-%ce%b49-tetrahydrocannabinol-provides-symptom-and-disease-modification-in-a-mouse-model-of-knee-osteoarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/oral-delivery-of-%ce%b49-tetrahydrocannabinol-provides-symptom-and-disease-modification-in-a-mouse-model-of-knee-osteoarthritis/