Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In Rheumatoid arthritis (RA), osteoclasts derived from CD14+ Monocytes are associated with a risk of progressive bone and joint destruction, which fundamentally impacts quality-of-life. Current RA treatments such as immunosuppressants, steroids, and biologics can in part ameliorate joint destruction and decrease the need for subsequent orthopaedic surgery. However, such treatments are far from perfect and knowledge of their mode-of-action in preventing osteoclast induced bone destruction is limited. IRL201805 is a novel biologic derived from Binding Immunoglobulin Protein (BiP) that has been developed for the treatment of RA ( P Eggleton, et al, J Cell Mol Med, 2023;27:322-339). In a Phase I/IIa clinical trial, a single IRL201805 intravenous infusion was demonstrated to improve Disease Activity Score 28 (DAS28) (B Kirkham, et al, Rheumatology, 2016;55:1993-2000). Moreover, IRL201805 was also shown to inhibit osteoclast function (V M Corrigall, et al, Immunology, 2009;128: 218-226). In this study, we have further investigated the capacity and mode-of-action of IRL201805 on receptor activator of nuclear factor-kB ligand (RANKL)-induced osteoclastogenesis in the context of RA patient derived CD14+ monocytes.
Methods: Pre- and mature osteoclasts were derived from CD14+CD16– monocytes, isolated from peripheral blood mononuclear cells of RA patients and healthy individuals, in in vitro cultures supplemented with macrophage-colony stimulating factor (M-CSF) and RANKL. Changes in gene transcript levels in preosteoclasts was examined by RNAseq, 24h after drug treatment.
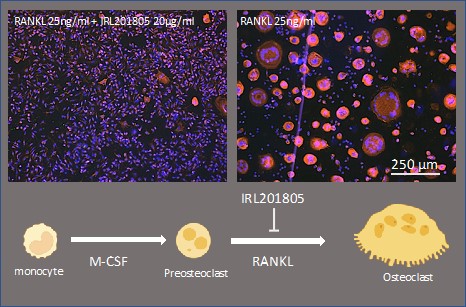
Results: IRL201805 reduced the numbers of osteoclasts generated from preosteoclasts derived from both RA patients (vehicle: 777±587 vs IRL201805: 102±12; n=5) and healthy controls (vehicle: 812±569 vs IRL201805: 148±179; n=5), as visualized by immunofluorescent TRAP staining(Figure 1). Notably, IRL201805 also inhibited actin ring formation. Transcriptional evaluation of pre-osteoclast exposure to IRL201805 revealed that several genes implicated in osteoclast differentiation were significantly downregulated (e.g. TNFSF11A (p< 0.002)) or upregulated (e.g. NFKBIZ (p< 0.01)). Moreover, several vacuolar ATPases (i.e. ATP6V0E2 (p< 0.001) and ATP6V1C1 (p< 0.01)), which play a role in acidification of osteoclasts and bone resorption, were strongly downregulated.
Conclusion: Our finding suggest that IRL201805 inhibits osteoclast differentiation via modulation of RANK/RANKL signaling. Notably, there is an impact on metabolic pathways such as V-ATPases, which are critical for bone resorption. This is particulary revelant, as antiresorptive drugs that inhibit V-ATPase activity in osteoclasts have become an area of development. Taken together, these findings underpin the need for further investigations in RA patients and upcoming clinical phase IIb RA trials.
To cite this abstract in AMA style:
Yamamura Y, Corrigall V, Ravanetti L, De Alba J, Foulkes R, Eggleton P, Goodyear C. Novel Biologic IRL201805 Inhibits Osteoclastogenesis in Monocytic Cells from RA Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/novel-biologic-irl201805-inhibits-osteoclastogenesis-in-monocytic-cells-from-ra-patients/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-biologic-irl201805-inhibits-osteoclastogenesis-in-monocytic-cells-from-ra-patients/