Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
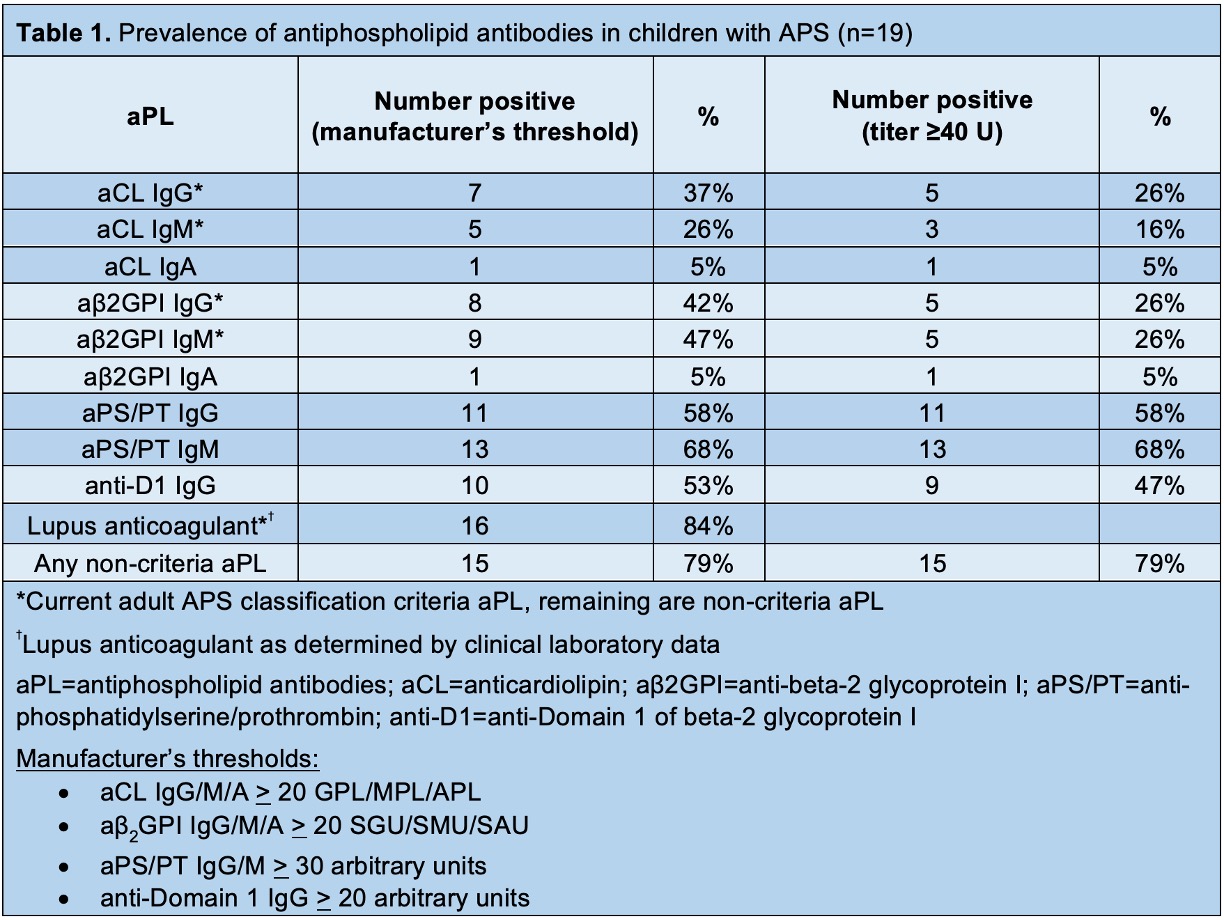
Background/Purpose: While classification criteria for pediatric APS are not yet available, recent research suggests pediatric APS patients are unique, and many present with extra-criteria manifestations as their primary clinical features. Criteria aPL may identify classical APS manifestations, but a clinically actionable biomarker for features unique to pediatric APS is not yet available. Here, we aimed to evaluate the presence, clinical associations, and potential mechanistic roles of non-criteria aPL and circulating NETs markers in pediatric APS patients.
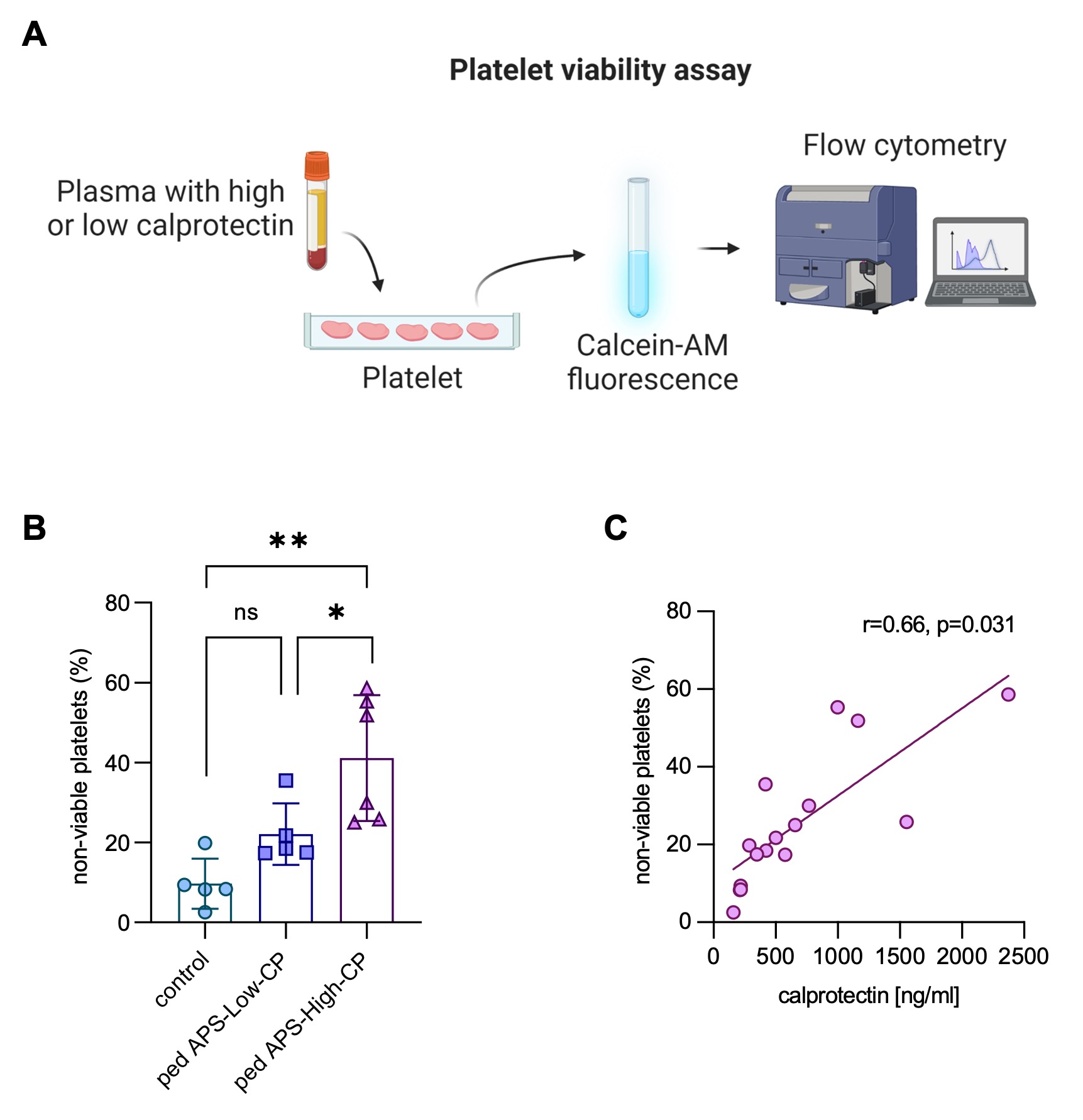
Methods: Children with APS (n=19), venous thrombosis without APS (VT, n=20), systemic lupus erythematosus without aPL (SLE, n=11), and healthy controls (n=13) were evaluated for nine different types of aPL by Werfen assays (Table 1). Calprotectin, a well-known marker of circulating neutrophil extracellular traps (NETs), was measured in plasma using the Human S100A8/S100A9 heterodimer DuoSet ELISA (R&D). Univariate logistic regression was used to identify potential clinical associations. A platelet viability dye-based assay was performed to assess the potential impact of calprotectin on platelet survival (Fig 2A).
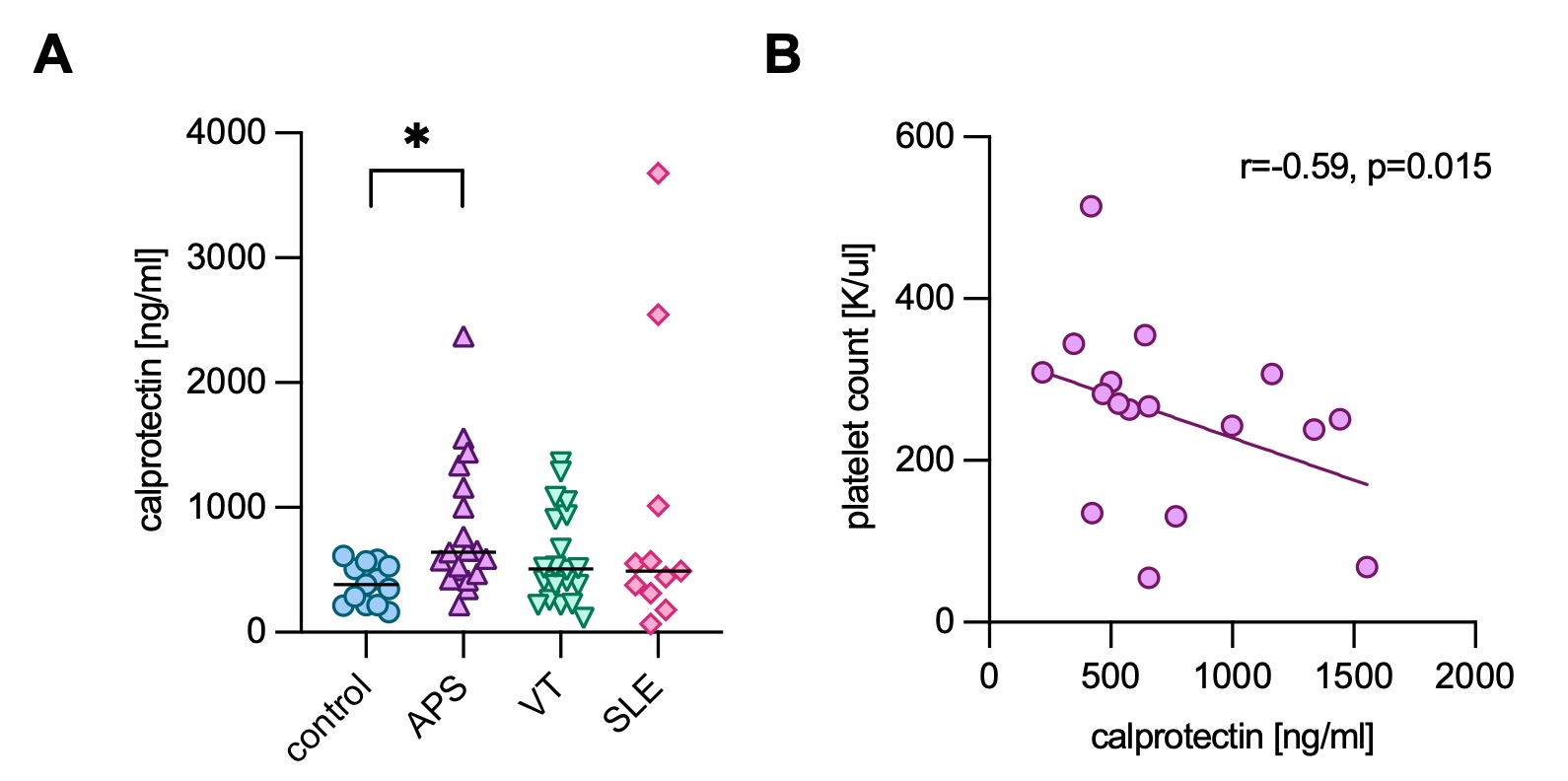
Results: Among the 19 pediatric APS patients (median age of 13 [range 3 to 18]), 74% were female, and 58% had primary APS. Most (89%) had experienced at least one venous thrombosis, and four had recurrent thrombotic events. The remaining two children with APS exhibited extra-criteria manifestations, persistent aPL, and a clinical diagnosis of APS. Almost half (47%) had at least one extra-criteria manifestation. Interestingly, 79% of pediatric APS patients had at least one non-criteria aPL at ≥40 units (Table 1). Aside from lupus anticoagulant, aPS/PT IgG and IgM were the most prevalent aPL followed by anti-D1 IgG. Univariate logistic regression demonstrated that positive anti-D1 (p=0.0109), positive aPS/PT IgG (p < 0.0001), and IgM (p < 0.0001) were significantly associated with venous thrombosis. Positive anti-D1 IgG (p=0.0116) and aPS/PT IgG (p=0.0116)/IgM (p=0.0321) were also associated with extra-criteria manifestations such as thrombocytopenia. Increased levels of calprotectin, which is a marker of NETs, were detected in children with APS (Fig 1A). Calprotectin levels correlated moderately with levels of aPS/PT IgM (r=0.49, p=0.0345) and absolute neutrophil count (r=0.63, p=0.0079), whereas they were negatively correlated with hemoglobin (r=-0.74, p=0.0189) and platelet count (r=-0.59, p=0.0150) (Fig 1B). Mechanistically, plasma from pediatric APS patients with high calprotectin levels impaired platelet survival in vitro in a dose-dependent manner (Fig 2A-B), and APS calprotectin levels correlated with decreased platelet viability (Fig 2C).
Conclusion: Our study suggests non-criteria aPL and circulating calprotectin were highly prevalent among pediatric APS patients and associated with extra-criteria manifestations more common in pediatric APS. The role of non-criteria aPL and calprotectin as clinically actionable biomarkers that might enable earlier diagnosis and inform targeted therapy for some pediatric aPL-positive patients warrants further evaluation.
To cite this abstract in AMA style:
Sloan E, Kmetova K, Somanathapura N, Kluge L, Chong E, Hoy C, Yalavarthi S, Madison J, Sarosh C, Walters L, Baisch J, Turnier J, Pascual V, Wright T, Knight J, Zia A, Zuo Y. Non-Criteria Antiphospholipid Antibodies and Calprotectin as Potential Biomarkers in Pediatric Antiphospholipid Syndrome [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/non-criteria-antiphospholipid-antibodies-and-calprotectin-as-potential-biomarkers-in-pediatric-antiphospholipid-syndrome/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/non-criteria-antiphospholipid-antibodies-and-calprotectin-as-potential-biomarkers-in-pediatric-antiphospholipid-syndrome/