Session Information
Date: Monday, October 27, 2025
Title: (0934–0954) Systemic Lupus Erythematosus – Animal Models Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is a multisystem autoimmune disease characterized by aberrant production of autoantibodies and chronic inflammation. Up to 80% of individuals with SLE experience cognitive, emotional, or behavioral symptoms, termed Neuropsychiatric Lupus (NPSLE). NPSLE cognitive impairment and mood disorders may be mediated by autoantibodies, with specific symptoms related to the varied targets of these autoantibodies and the region of blood brain barrier (BBB) injury. We use a murine model of NPSLE induced by anti-double-stranded DNA (dsDNA) antibodies (DNRAbs), which cross-react with the excitatory neuronal N-methyl D-aspartate receptor (NMDAR) and facilitate excitotoxic neuronal death. These antibodies are present in approximately 30% of patients with SLE and at a higher rate in those with NPSLE. Prior research in our lab has shown that epinephrine administration to DNRAb+ mice leads to acute neuronal excitotoxicity in the amygdala. Investigation of the chronic phenotype in the amygdala may lead to insights into the prevalence of persistent mood and anxiety disorders experienced by patients.
Methods: Female B6H2d mice used in this study are immunized with DWEYS peptide on a polylysine backbone to produce DNRAbs or with the polylysine backbone as a control. PET studies using 11C-AIB tracer are used for BBB permeability with epinephrine administration. Unbiased stereology and immunohistochemistry are used to identify cell type morphology, protein expression, and quantify neuronal loss. Behavioral testing includes object place memory, elevated plus maze, and fear conditioning. Mann-Whitney and T-tests are used for statistical analysis between groups.
Results: In this study, we characterize the chronic effects of DNRAb antibody penetration into the brain after systemic administration of epinephrine. We show that BBB permeability due to epinephrine is selective for the amygdala using 11C-AIB PET imaging, resulting in antibody deposition into the amygdala. We observe significant neuronal loss in the basolateral and lateral nuclei of the amygdala in DNRAb+ compared to DNRAb- mice, correlating with amygdala-related behavioral findings in anxiety testing. Finally we observe an abnormal morphology and lipid oxidation suggestive of axonal degeneration in surviving neurons.
Conclusion: Interestingly, the DNRAb-mediated neuronal pathology in the amygdala, which we currently hypothesize is due to ferroptosis, differs from that in the hippocampus, which is characterized by chronic microglial-dependent synaptic elimination and loss of dendritic arborization. Further investigation will focus on circuit disruption and detailed analysis of mechanisms of chronic neuronal damage. This work expands our understanding of the neuropathological mechanisms which can underly psychiatric symptoms in NPSLE.
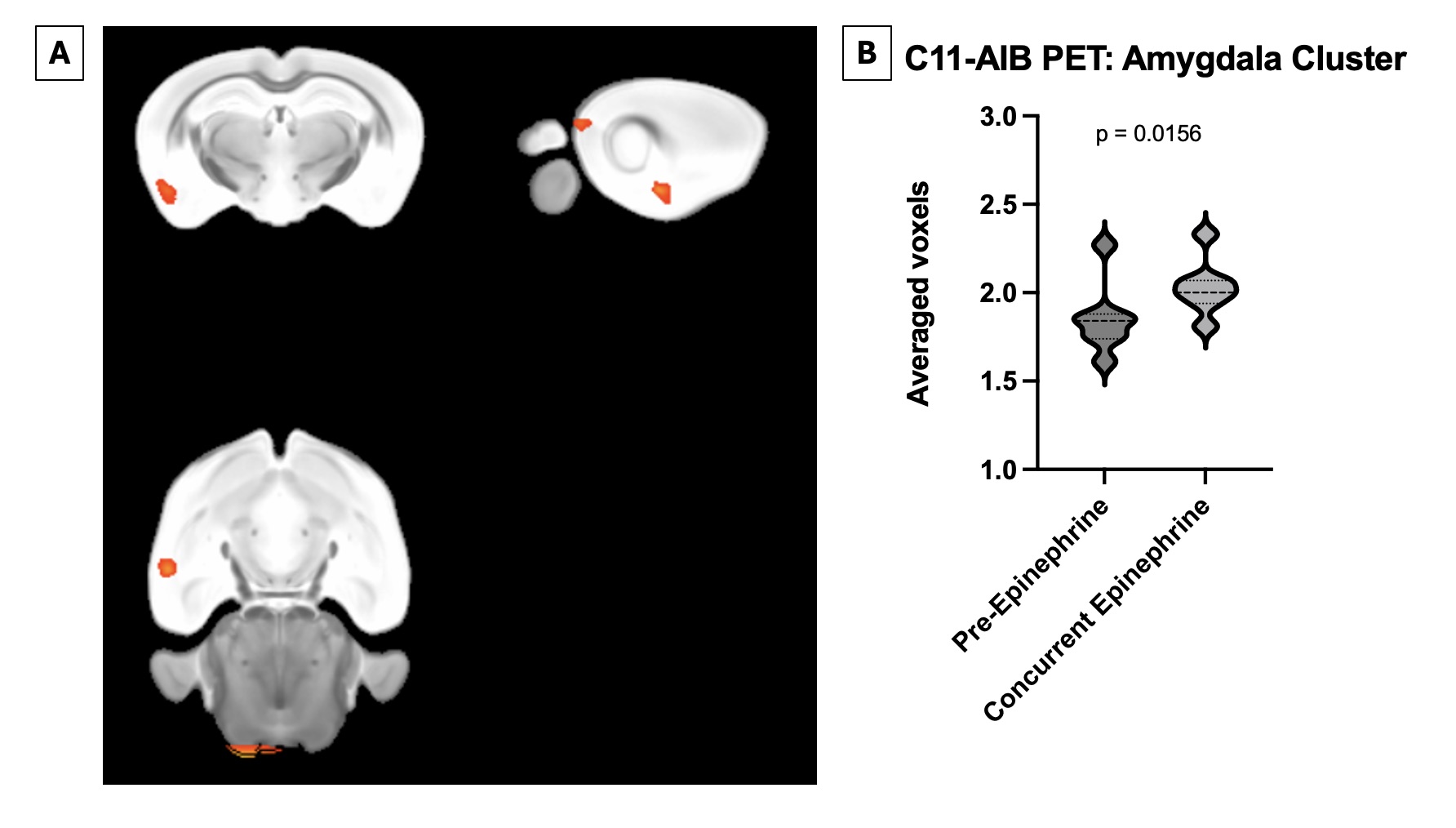 Figure 1: Epinephrine induces a specific blood brain barrier breach in the area of the amygdala in both DNRAb+ and control animals. A) Visualization of thresholded C11-AIB PET scan comparing both DNRAb+ and DNRAb- animals pre-epinephrine and post-epinephrine administration. B) Quantification of C11-AIB PET scan average of voxels in the amygdala, normalized by global mean in each mouse comparing both DNRAb+ and DNRAb- animals pre-epinephrine and post-epinephrine administration (p=0.0156, paired t test, n=8).
Figure 1: Epinephrine induces a specific blood brain barrier breach in the area of the amygdala in both DNRAb+ and control animals. A) Visualization of thresholded C11-AIB PET scan comparing both DNRAb+ and DNRAb- animals pre-epinephrine and post-epinephrine administration. B) Quantification of C11-AIB PET scan average of voxels in the amygdala, normalized by global mean in each mouse comparing both DNRAb+ and DNRAb- animals pre-epinephrine and post-epinephrine administration (p=0.0156, paired t test, n=8).
.jpg) Figure 2: DNRAb+ mice demonstrate significant neuronal loss in multiple regions of the amygdala compared to control mice. A) Representative images of immunofluorescence confocal imaging of LAD and BLA of DNRAb- and DNRAb+ mice. B) Cell counts per area using unbiased stereology of the lateral amygdala nuclei in DNRAb- and DNRAb+ mice. C) Cell counts per area using unbiased stereology of the basolateral amygdala nuclei in DNRAb- and DNRAb+ mice.
Figure 2: DNRAb+ mice demonstrate significant neuronal loss in multiple regions of the amygdala compared to control mice. A) Representative images of immunofluorescence confocal imaging of LAD and BLA of DNRAb- and DNRAb+ mice. B) Cell counts per area using unbiased stereology of the lateral amygdala nuclei in DNRAb- and DNRAb+ mice. C) Cell counts per area using unbiased stereology of the basolateral amygdala nuclei in DNRAb- and DNRAb+ mice.
To cite this abstract in AMA style:
Weissman-Tsukamoto R, Volpe B, Ibic Z, Carroll K, Vo A, Kowal C, Diamond B. NMDAR Autoantibody-Induced Neuronal Damage in the Amygdala Mediates Mood and Anxiety Disorders in a Model of Neuropsychiatric Lupus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/nmdar-autoantibody-induced-neuronal-damage-in-the-amygdala-mediates-mood-and-anxiety-disorders-in-a-model-of-neuropsychiatric-lupus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nmdar-autoantibody-induced-neuronal-damage-in-the-amygdala-mediates-mood-and-anxiety-disorders-in-a-model-of-neuropsychiatric-lupus/
